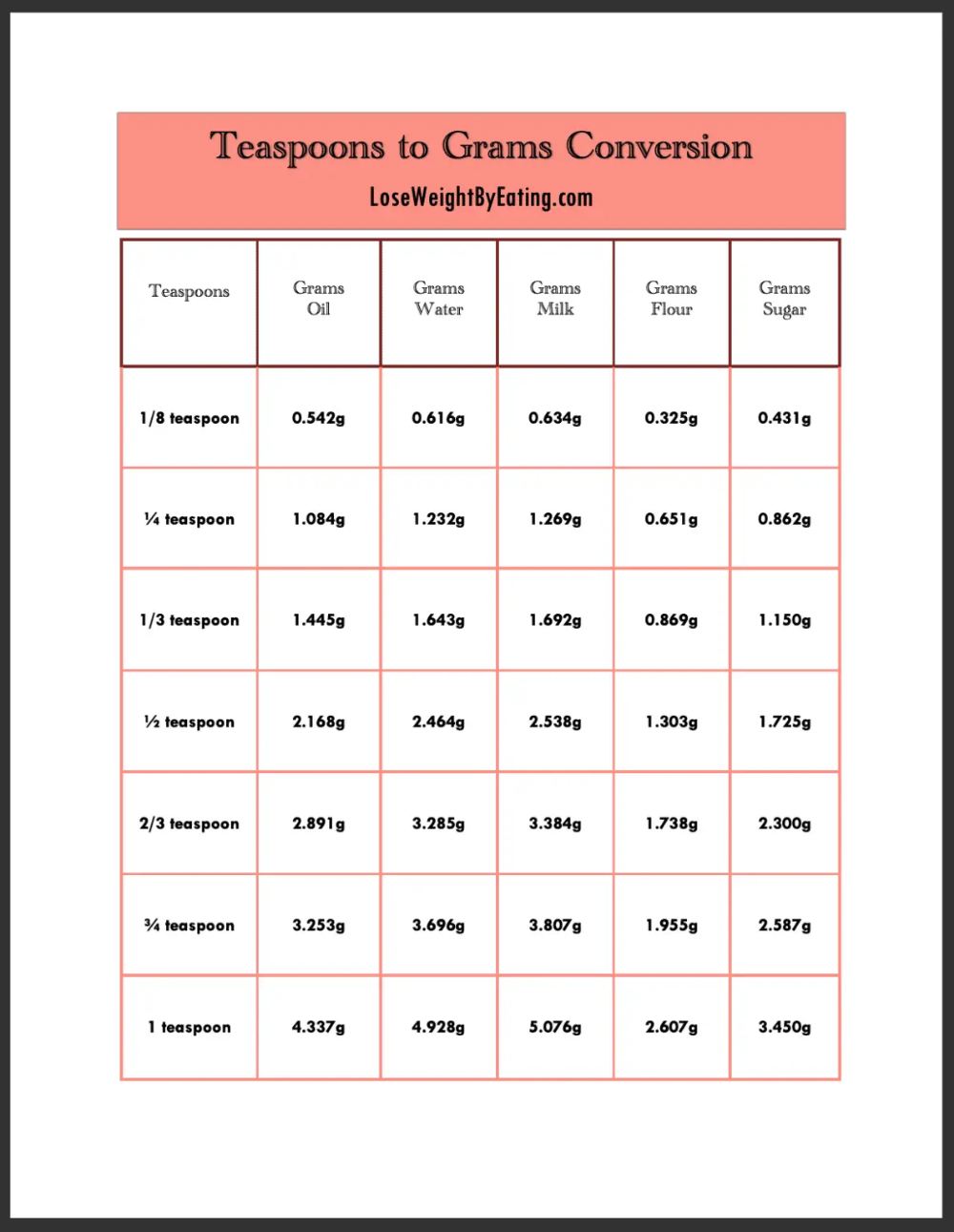
The concept of measuring 1 gram in a liter is fundamental in understanding density and concentration in physics and chemistry. To put it simply, 1 gram per liter (g/L) is a unit of measurement that represents the mass of a substance per unit volume of a solution or mixture. This unit is crucial in various scientific and industrial applications, including water treatment, chemical engineering, and pharmaceutical manufacturing.
Density and Concentration
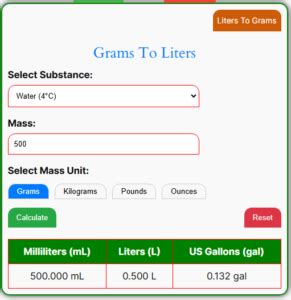
Density is defined as mass per unit volume of a substance. When we say 1 gram in a liter, we’re essentially talking about the density of a solution where 1 gram of a substance is dissolved in 1 liter of a solvent. This measurement is equivalent to 1 kilogram per cubic meter (kg/m³) since 1 liter equals 0.001 cubic meters and 1 gram equals 0.001 kilograms. Understanding density is vital because it influences how substances interact and behave under different conditions.
Practical Applications
In practical terms, measuring 1 gram in a liter is essential for preparing solutions with precise concentrations. For example, in laboratories, scientists often need to prepare solutions with exact concentrations for experiments. In water treatment facilities, knowing the concentration of substances like chlorine or fluoride is critical for ensuring the water is safe for consumption. Similarly, in pharmaceuticals, precise concentrations of active ingredients are necessary for efficacy and safety.
| Substance | Concentration (g/L) | Application |
|---|---|---|
| Sodium Chloride (NaCl) | 3.5 | Seawater |
| Sucrose (C12H22O11) | 500 | Syrups |
| Chlorine (Cl2) | 0.5-1.0 | Water Disinfection |
Calculations and Conversions
Calculating concentrations in terms of grams per liter is straightforward. If you know the mass of the solute and the volume of the solution, you can calculate the concentration using the formula: Concentration (g/L) = Mass of solute (g) / Volume of solution (L). Conversely, if you know the concentration and the volume of the solution, you can calculate the mass of the solute needed.
Conversion Factors
Understanding conversion factors is also important. For instance, to convert milligrams per liter (mg/L) to grams per liter, you divide by 1000 since there are 1000 milligrams in a gram. This kind of conversion is common in environmental science and engineering, where concentrations of pollutants are often measured in parts per million (ppm) or parts per billion (ppb), which can be related to grams per liter.
Key Points
- 1 gram in a liter is equivalent to 1 kilogram per cubic meter.
- This measurement is crucial for understanding density and concentration.
- Practical applications include laboratory preparations, water treatment, and pharmaceutical manufacturing.
- Concentration affects the physical and chemical properties of substances.
- Calculations and conversions between different units of concentration are essential for precise applications.
In conclusion, understanding and working with concentrations like 1 gram in a liter is fundamental across various scientific disciplines and industries. It requires not just knowledge of the units and how to calculate them but also an appreciation for how concentration affects the behavior and properties of substances.
What does 1 gram in a liter signify?
+It signifies the concentration or density of a substance in a solution, where 1 gram of the substance is dissolved in 1 liter of the solvent.
Why is measuring 1 gram in a liter important?
+It’s crucial for preparing solutions with precise concentrations, which is essential in laboratories, water treatment, and pharmaceutical manufacturing.
How do you calculate concentration in grams per liter?
+You calculate it by dividing the mass of the solute by the volume of the solution: Concentration (g/L) = Mass of solute (g) / Volume of solution (L).