The concept of measuring the amount of a substance, such as a chemical or a solute, in a given volume of a solution, like water, is fundamental in chemistry and various scientific disciplines. One common method of expressing concentration is in terms of ounces per gallon. To understand how many ounces are in a gallon of water, we need to delve into the basics of measurement units and conversion factors.
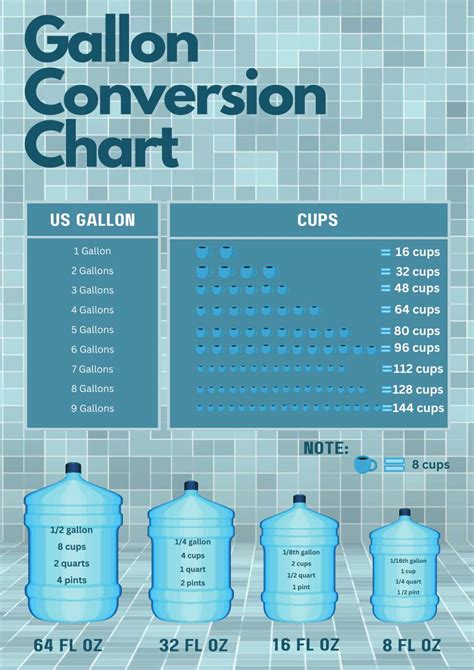
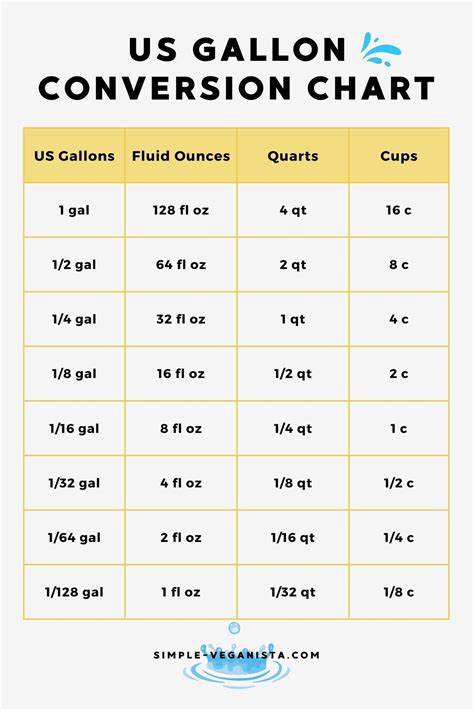
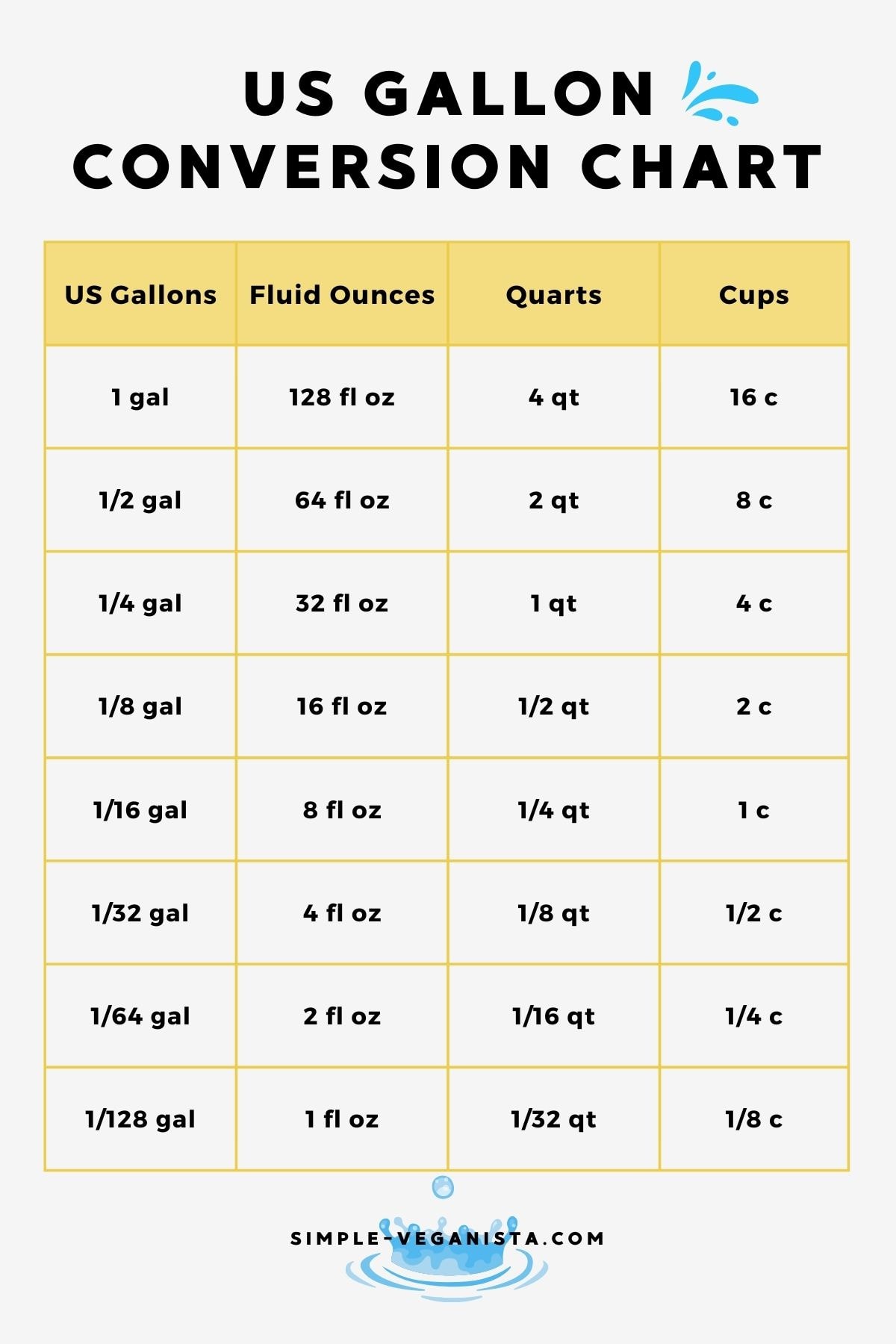
A gallon is a unit of volume, primarily used in the United States, and it equals 128 fluid ounces. This conversion factor is critical when calculating the amount of a substance in a solution. For instance, if we were to consider a gallon of water, which is purely water, we wouldn't typically express its concentration in ounces of water per gallon because the entire gallon is water. However, understanding this relationship is essential for more complex calculations involving solutions.
Key Points
- 1 gallon of water equals 128 fluid ounces of water.
- The concept of concentration in solutions is crucial for scientific and practical applications.
- Understanding conversion factors is key to accurately calculating the amount of a substance in a given volume.
- In scientific contexts, the concentration of a solution can be expressed in various units, including parts per million (ppm), parts per billion (ppb), molarity, and more.
- The specific gravity of water is approximately 1, which means that 1 gallon of water weighs about 8.34 pounds, given that 1 pound of water is approximately equal to 0.12004 gallons.
Understanding Concentration and Volume
Concentration refers to the amount of a substance (solute) per unit volume or mass of a mixture or solution. In the context of a gallon of water, if we were discussing the water itself, we wouldn’t typically talk about its concentration since it’s the solvent making up the entirety of the solution. However, when additives or solutes are introduced into the water, understanding their concentration becomes crucial. This is where the concept of ounces per gallon comes into play, especially in applications like water treatment, where certain chemicals need to be added in precise amounts to achieve desired effects.
Calculating Concentration
To calculate the concentration of a solution in terms of ounces per gallon, one must know the weight or volume of the solute and the volume of the solution. For example, if you have a chemical that needs to be added to water at a concentration of 1 ounce per gallon, you would add 1 ounce of the chemical to 1 gallon of water. This calculation becomes more complex when dealing with different units of measurement for the solute and the solvent or when the specific gravity of the solute differs significantly from that of water.
| Unit of Measurement | Conversion Factor |
|---|---|
| Fluid Ounces to Gallons | 1 gallon = 128 fluid ounces |
| Pounds to Gallons (Water) | 1 gallon of water ≈ 8.34 pounds |

Practical Applications and Considerations
In real-world scenarios, such as water treatment, agricultural applications, and manufacturing processes, the ability to accurately measure and calculate concentrations is essential. This involves not only understanding the conversion factors between different units of measurement but also being aware of the physical and chemical properties of the substances involved. For instance, the solubility of a substance in water, its reactivity, and its potential environmental impact are all critical factors that must be considered when preparing solutions for various applications.
Furthermore, regulations and standards often dictate the acceptable concentrations of certain substances in water or other solvents, especially in contexts where human health or environmental safety is a concern. Compliance with these regulations requires meticulous attention to detail in measurement and calculation, as well as a deep understanding of the chemical and physical principles governing solution behavior.
Environmental and Health Implications
The concentration of substances in water can have significant environmental and health implications. For example, high concentrations of certain pollutants in waterways can harm aquatic life and contaminate the food chain. Similarly, in drinking water, the presence of contaminants above certain concentration levels can pose serious health risks to humans. Therefore, understanding and controlling concentrations in solutions is not just a matter of scientific interest but also of public health and environmental protection.
What is the conversion factor between fluid ounces and gallons?
+1 gallon is equal to 128 fluid ounces.
Why is understanding concentration in solutions important?
+Understanding concentration is crucial for scientific applications, industrial processes, and environmental protection, as it affects the properties and behavior of solutions and can have significant implications for health and safety.
How does the specific gravity of a substance affect its concentration in a solution?
+The specific gravity of a substance, which is the ratio of its density to the density of water, can affect how it dissolves in water and its concentration in a solution. Substances with a specific gravity close to that of water (approximately 1) will behave differently than those with significantly higher or lower specific gravities.
In conclusion, the concept of ounces in a gallon of water, while straightforward in terms of simple conversion, opens up a broader discussion on the importance of concentration in solutions. Whether in scientific research, industrial applications, or environmental protection, understanding how to calculate and control concentrations is vital. By grasping the fundamental principles of measurement, conversion, and the physical properties of substances, individuals can better navigate the complex world of solutions and contribute to advancements in various fields.