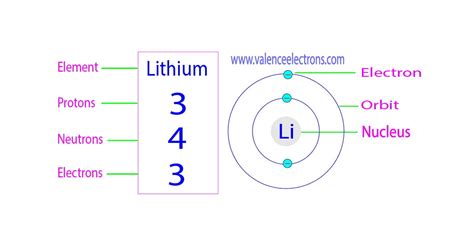
Lithium, with its atomic number of 3, is a lightweight metal that has garnered significant attention due to its unique properties and applications, particularly in the realm of battery technology and nuclear physics. The proton, a fundamental particle with a positive charge, plays a crucial role in defining the atomic structure of elements, including lithium. When considering lithium protons, several key facts emerge that highlight the intricacies of atomic physics and the distinctive characteristics of lithium.
Key Points
- Lithium has 3 protons in its atomic nucleus, which defines its atomic number and places it in the first column of the periodic table.
- The number of protons in an atom's nucleus determines the element's identity in the periodic table, with each unique number of protons corresponding to a different element.
- Lithium's isotopes, which have varying numbers of neutrons but the same number of protons, exhibit different physical properties and are used in different applications, ranging from nuclear reactions to advanced battery technologies.
- The interaction between protons and other subatomic particles, such as electrons and neutrons, is crucial for understanding the chemical and physical properties of lithium and its compounds.
- Lithium's reactivity, particularly its tendency to lose one electron to form a positive ion, is influenced by its atomic structure, including the arrangement of its protons and electrons.
Understanding Lithium Protons and Atomic Structure
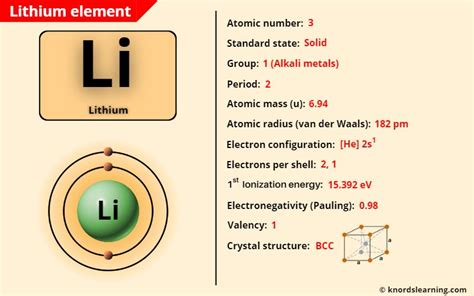
The atomic structure of lithium, like all elements, is characterized by its atomic number, which is the number of protons found in the nucleus of an atom. For lithium, this number is 3, meaning that every lithium atom has 3 protons. This fundamental aspect of lithium’s structure is crucial for its chemical properties and reactivity, as the number of protons (and thus the number of electrons in a neutral atom) determines how lithium interacts with other elements.
Lithium Isotopes and Their Applications
Lithium, with its 3 protons, can exist in various isotopic forms, which differ in the number of neutrons in the nucleus. The two most common isotopes of lithium are lithium-6 and lithium-7, with 3 protons and either 3 or 4 neutrons, respectively. These isotopes have different physical properties and uses. For instance, lithium-6 is used in nuclear applications due to its ability to absorb neutrons, while lithium-7 is more abundant and is used in various chemical applications, including the production of lubricating greases and in the manufacture of ceramics and glass.
| Isotope | Number of Neutrons | Applications |
|---|---|---|
| Lithium-6 | 3 | Nuclear reactions, production of tritium |
| Lithium-7 | 4 | Chemical applications, ceramics, glass manufacturing |

Chemical Properties Influenced by Protons
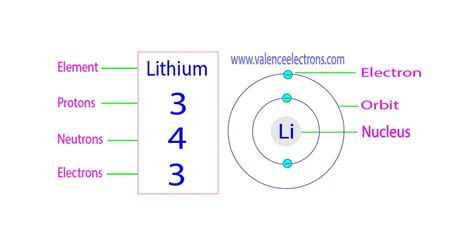
The chemical properties of lithium, such as its reactivity and the types of compounds it can form, are significantly influenced by its atomic structure, particularly the number of protons and electrons. Lithium’s tendency to lose one electron to form a Li+ ion is a direct result of its electronic configuration, which is determined by the number of protons in the nucleus. This reactivity makes lithium useful in batteries, where the movement of lithium ions facilitates the flow of electrical current.
Nuclear Stability and Proton-Neutron Interactions
The stability of lithium’s nucleus, comprising 3 protons and a variable number of neutrons, is a function of the strong nuclear force that binds protons and neutrons together. The interaction between protons and neutrons is critical for understanding the nuclear stability of lithium and its isotopes. This stability, in turn, affects the element’s radioactivity and its potential applications in nuclear physics and technology.
In conclusion, the study of lithium protons offers insights into the fundamental nature of atomic structure and its implications for chemical and physical properties. By understanding the role of protons in defining an element's identity and behavior, scientists can better appreciate the complexities of the periodic table and the unique characteristics of elements like lithium.
What is the significance of the number of protons in an atom of lithium?
+The number of protons in a lithium atom, which is 3, determines its atomic number and thus its position in the periodic table. This number is fundamental to the element’s chemical properties and its ability to form compounds with other elements.
How do the isotopes of lithium differ, and what are their applications?
+Lithium isotopes differ in the number of neutrons in their nuclei. Lithium-6, with 3 neutrons, is used in nuclear applications, while lithium-7, with 4 neutrons, is used in chemical applications, including the production of ceramics and glass. The choice of isotope depends on the specific requirements of the application.
What role do protons play in the chemical reactivity of lithium?
+The protons in a lithium atom determine its electronic configuration, which in turn influences its chemical reactivity. Lithium’s tendency to lose one electron to form a positive ion is a result of its electronic structure, which is directly related to the number of protons in the nucleus.