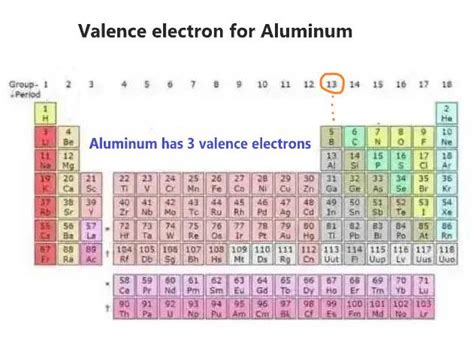
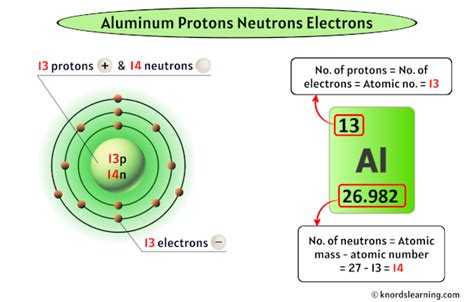
Aluminum, commonly referred to as Al, is a chemical element with the atomic number 13. It is a soft, silvery-white, ductile metal in the boron group. By understanding how aluminum has electrons, we can delve into its atomic structure and properties. The arrangement of electrons in an atom is crucial for determining its chemical behavior and reactivity. In the case of aluminum, its electron configuration is 1s² 2s² 2p⁶ 3s² 3p¹, indicating how its 13 electrons are distributed across different energy levels.
Understanding Electron Configuration
The electron configuration of aluminum shows that it has three energy levels or shells. The first shell (1s) is fully occupied with two electrons, the second shell (2s and 2p) is also fully occupied with eight electrons, and the third shell starts to fill with two electrons in the 3s orbital and one electron in the 3p orbital. This configuration is significant because it influences aluminum’s chemical properties, such as its tendency to lose three electrons to form a stable ion with a +3 charge, thus achieving a noble gas configuration similar to that of neon.
Electron Arrangement and Chemical Properties
The electron arrangement in aluminum is key to its reactivity. With three electrons in its outermost shell, aluminum readily forms compounds where it donates these electrons, exhibiting a +3 oxidation state. This is evident in its oxides, halides, and other compounds where aluminum acts as a metal, losing electrons to form a cation. The electron configuration also explains aluminum’s position in the periodic table and its relation to other elements in the same group and period.
| Energy Level | Electron Orbitals | Number of Electrons |
|---|---|---|
| 1st | 1s | 2 |
| 2nd | 2s, 2p | 8 |
| 3rd | 3s, 3p | 3 |
Key Points
- Aluminum's atomic number is 13, with an electron configuration of 1s² 2s² 2p⁶ 3s² 3p¹.
- The electron arrangement influences aluminum's chemical properties, including its tendency to form a +3 ion.
- Understanding the electron configuration is crucial for predicting aluminum's reactivity and its position in the periodic table.
- The outermost energy level of aluminum contains three electrons, which are readily lost to form compounds.
- The electron configuration explains aluminum's behavior in different chemical environments and its utility in various applications.
Applications and Properties
Aluminum’s unique electron configuration contributes to its low density, high ductility, and resistance to corrosion, making it a versatile metal for a wide range of applications. From construction and transportation to packaging and electronics, aluminum’s properties, which are fundamentally linked to its electron arrangement, make it an indispensable material in modern society.
Environmental and Health Considerations
While aluminum is generally considered safe and is even a part of some cooking utensils and food packaging, its production and disposal have environmental implications. The extraction of aluminum from bauxite, its primary ore, and the smelting process require significant amounts of energy, contributing to greenhouse gas emissions. Furthermore, improper disposal of aluminum products can lead to environmental pollution. Understanding the full lifecycle of aluminum, from its atomic structure to its applications and disposal, is essential for mitigating its environmental impact.
What is the electron configuration of aluminum?
+The electron configuration of aluminum is 1s² 2s² 2p⁶ 3s² 3p¹, which dictates its chemical properties and reactivity.
Why does aluminum tend to lose three electrons?
+Aluminum loses three electrons to achieve a stable noble gas configuration, similar to that of neon, which has a full outer energy level.
What are some common applications of aluminum?
+Aluminum is used in construction, transportation (including aircraft and vehicles), packaging, and electronics due to its unique combination of properties such as low density, corrosion resistance, and ductility.
In conclusion, the electron configuration of aluminum plays a pivotal role in determining its chemical and physical properties, which in turn influence its wide range of applications. From its reactivity and tendency to form ions to its use in everyday products, understanding the electron arrangement of aluminum is essential for appreciating its significance in science and industry. As research and technology continue to evolve, the importance of aluminum and the understanding of its atomic structure will remain fundamental to advancements in materials science and beyond.