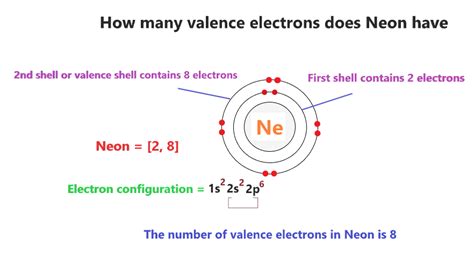
The concept of 8 valence electrons is a fundamental principle in chemistry, particularly in the realm of atomic structure and chemical bonding. Valence electrons are the electrons in the outermost shell of an atom, and they play a crucial role in determining the chemical properties of an element. The notion that atoms tend to gain, lose, or share electrons to achieve a full outer shell with 8 valence electrons is a cornerstone of chemistry, explaining why certain elements form compounds with specific stoichiometries and exhibit characteristic reactivities.
The Octet Rule and Its Significance
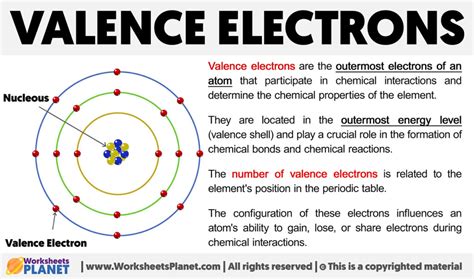
The octet rule, which states that atoms strive to have 8 electrons in their valence shell, is a simplification of the more complex electron configuration rules. This rule is particularly useful for understanding the behavior of main-group elements, which include the s-block and p-block elements of the periodic table. By achieving a full outer shell with 8 valence electrons, atoms can attain a noble gas configuration, which is energetically stable. This stability is due to the full outer shell having a particularly low energy state, making it less reactive.
Formation of Ions and Compounds
Atoms that do not naturally have 8 valence electrons will often engage in chemical reactions to achieve this stable configuration. For instance, metals tend to lose electrons to form cations, while nonmetals tend to gain electrons to form anions. The combination of cations and anions leads to the formation of ionic compounds. In covalent compounds, atoms share electrons to achieve a full outer shell, illustrating the flexibility of chemical bonding in achieving the octet rule. This principle underlies the vast diversity of chemical compounds and their properties.
| Element | Number of Valence Electrons | Stability |
|---|---|---|
| Neon (Ne) | 8 | Highly Stable (Noble Gas) |
| Sodium (Na) | 1 | Reactive (Metal) |
| Fluorine (F) | 7 | Highly Reactive (Nonmetal) |
| Oxygen (O) | 6 | Reactive (Nonmetal) |
Key Points
- The octet rule explains why atoms tend to gain, lose, or share electrons to achieve a stable configuration with 8 valence electrons.
- This principle is particularly relevant for main-group elements and underlies the formation of both ionic and covalent compounds.
- Achieving a full outer shell with 8 valence electrons leads to a noble gas configuration, which is energetically stable and less reactive.
- Understanding the octet rule is essential for predicting chemical properties, reactivities, and the formation of compounds.
- Lewis structures, which represent the distribution of electrons in molecules, rely heavily on the concept of 8 valence electrons for predicting molecular geometry and reactivity.
Applications and Limitations
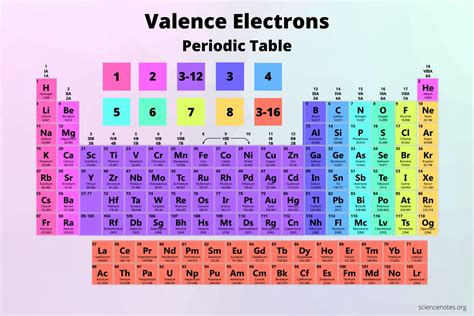
The principle of 8 valence electrons has numerous applications in chemistry, from predicting the reactivity of elements to understanding the properties of molecules. However, it is also important to recognize its limitations. The octet rule does not apply universally, particularly for transition metals and some heavier elements, where the expanded d and f orbitals can accommodate more than 8 electrons in the valence shell. Additionally, molecules like BF3 and SO3, which do not obey the octet rule due to the presence of less than 8 valence electrons around the central atom, highlight exceptions to this principle.
Evolutionary Developments and Historical Context
Historically, the development of the octet rule was closely tied to the discovery of the periodic table and the understanding of electron configuration. Over time, as more was learned about atomic structure and quantum mechanics, the principle has been refined. Today, it remains a fundamental concept in introductory chemistry, serving as a stepping stone for more advanced theories and models of chemical bonding and reactivity.
What is the significance of the octet rule in chemistry?
+The octet rule is significant because it explains why atoms react to form compounds and how they achieve a stable electron configuration, akin to the noble gases, which are chemically inert.
Are there exceptions to the octet rule?
+Yes, there are exceptions, particularly among transition metals and heavier elements where expanded orbitals can accommodate more electrons, and in molecules like BF3 and SO3, which have less than 8 valence electrons around the central atom.
How does the octet rule apply to ionic and covalent compounds?
+In ionic compounds, atoms gain or lose electrons to achieve the octet, forming ions that then attract each other. In covalent compounds, atoms share electrons to achieve a full outer shell, illustrating the principle's role in explaining chemical bonding.
In conclusion, the principle of 8 valence electrons is a foundational concept in chemistry, underpinning our understanding of chemical reactivity, compound formation, and molecular properties. While it has its limitations and exceptions, particularly with transition metals and heavier elements, its significance in explaining the behavior of main-group elements and in the construction of Lewis structures cannot be overstated. As our understanding of chemistry continues to evolve, the octet rule remains a crucial teaching tool and a cornerstone of chemical theory, providing a basis for more advanced studies in chemistry and related fields.