Molality is a measure of the concentration of a solution, defined as the number of moles of solute per kilogram of solvent. Calculating molality can be a straightforward process if you understand the basic principles and have the necessary information. In this article, we will explore the concept of molality, its importance, and provide a step-by-step guide on how to calculate it easily.
Key Points
- Molality is defined as the number of moles of solute per kilogram of solvent.
- The formula to calculate molality is: molality = moles of solute / mass of solvent (in kg).
- Understanding the concept of molality is crucial in various fields, including chemistry, physics, and engineering.
- Molality is an essential parameter in determining the properties of a solution, such as its freezing point, boiling point, and vapor pressure.
- Calculating molality requires accurate measurements of the mass of the solute and solvent, as well as the molar mass of the solute.
Understanding Molality
Molality is an important concept in chemistry and physics, as it helps us understand the properties of a solution. It is a measure of the concentration of a solution, which is essential in determining its behavior and characteristics. Molality is defined as the number of moles of solute per kilogram of solvent. For example, if you have a solution containing 2 moles of sugar (solute) dissolved in 1 kilogram of water (solvent), the molality of the solution would be 2 moles/kg.
Calculating Molality
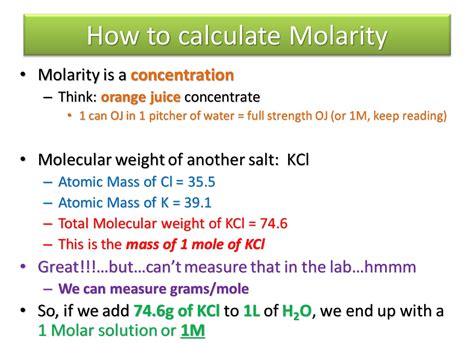
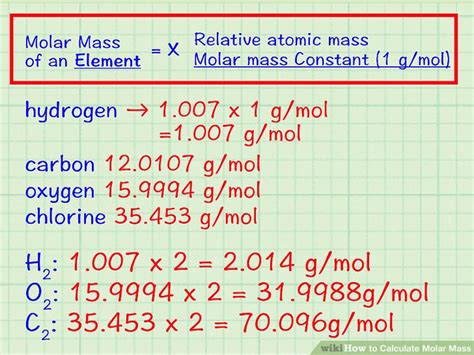
Calculating molality is a straightforward process that requires accurate measurements of the mass of the solute and solvent, as well as the molar mass of the solute. The formula to calculate molality is: molality = moles of solute / mass of solvent (in kg). To calculate the number of moles of solute, you need to know the mass of the solute and its molar mass. The molar mass is the mass of one mole of a substance, usually expressed in units of grams per mole (g/mol).
For example, let's say you have a solution containing 50 grams of sodium chloride (NaCl) dissolved in 200 grams of water. To calculate the molality of the solution, you need to first calculate the number of moles of NaCl. The molar mass of NaCl is 58.44 g/mol. Using the formula: moles = mass / molar mass, you can calculate the number of moles of NaCl as follows: moles = 50 g / 58.44 g/mol = 0.856 moles. Next, you need to calculate the mass of the solvent (water) in kilograms. Since the mass of water is 200 grams, you can convert it to kilograms by dividing by 1000: mass of water = 200 g / 1000 = 0.2 kg. Finally, you can calculate the molality of the solution using the formula: molality = moles of solute / mass of solvent = 0.856 moles / 0.2 kg = 4.28 mol/kg.
| Substance | Mass (g) | Molar Mass (g/mol) | Moles |
|---|---|---|---|
| Sodium Chloride (NaCl) | 50 | 58.44 | 0.856 |
| Water | 200 | - | - |
| Molality | - | - | 4.28 mol/kg |

Importance of Molality
Molality is an essential parameter in determining the properties of a solution, such as its freezing point, boiling point, and vapor pressure. It is also crucial in understanding the behavior of solutions in various industrial and scientific applications. For example, in the production of pharmaceuticals, molality is used to determine the concentration of active ingredients in a solution. In the field of engineering, molality is used to design and optimize systems that involve the use of solutions, such as in the production of fuels, chemicals, and other materials.
Applications of Molality
Molality has a wide range of applications in various fields, including chemistry, physics, engineering, and biology. It is used to determine the concentration of solutions, which is essential in understanding their properties and behavior. Some of the key applications of molality include:
- Determining the freezing point and boiling point of a solution
- Calculating the vapor pressure of a solution
- Designing and optimizing systems that involve the use of solutions
- Producing pharmaceuticals and other chemicals
- Understanding the behavior of solutions in various industrial and scientific applications
What is the difference between molality and molarity?
+Molality and molarity are both measures of concentration, but they differ in the units used to express the amount of solute and solvent. Molality is defined as the number of moles of solute per kilogram of solvent, while molarity is defined as the number of moles of solute per liter of solution.
How do I calculate the molality of a solution if I don't know the molar mass of the solute?
+If you don't know the molar mass of the solute, you can look it up in a reference table or calculate it using the atomic masses of the elements that make up the solute.
What are some common units used to express molality?
+Some common units used to express molality include moles per kilogram (mol/kg), moles per liter (mol/L), and grams per liter (g/L).
In conclusion, calculating molality is a straightforward process that requires accurate measurements of the mass of the solute and solvent, as well as the molar mass of the solute. Understanding the concept of molality is crucial in various fields, including chemistry, physics, and engineering, as it helps us determine the properties of a solution and its behavior. By following the steps outlined in this article and using the correct formula, you can easily calculate the molality of a solution and gain a deeper understanding of its properties and applications.