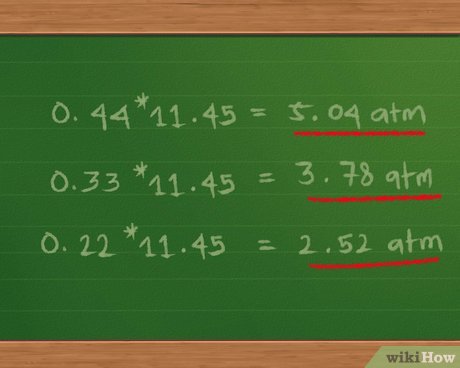Calculating partial pressure is a crucial concept in chemistry and physics, particularly in the study of gases. Partial pressure refers to the pressure exerted by a single component of a mixture of gases. It is a fundamental principle in understanding the behavior of gases and is widely applied in various fields, including chemistry, physics, engineering, and environmental science. In this article, we will delve into the concept of partial pressure, its significance, and explore five ways to calculate it, providing a comprehensive understanding of the subject.
Key Points
- Understanding the concept of partial pressure and its importance in gas mixtures
- Learning the different methods to calculate partial pressure, including Dalton's Law, the Ideal Gas Law, and others
- Applying partial pressure calculations in real-world scenarios, such as scuba diving and atmospheric science
- Recognizing the limitations and assumptions of each calculation method
- Developing problem-solving skills through practice exercises and examples
Introduction to Partial Pressure
Partial pressure is defined as the pressure that a single component of a mixture of gases would exert if it alone occupied the entire volume of the mixture at the same temperature. The concept of partial pressure is based on Dalton’s Law, which states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of each individual gas. This law is a fundamental principle in understanding the behavior of gases and is crucial in calculating partial pressures.
Dalton’s Law and Partial Pressure
Dalton’s Law provides a straightforward method to calculate partial pressure. According to this law, the partial pressure of a gas in a mixture is given by the formula: P_i = (n_i / n_total) * P_total, where P_i is the partial pressure of the gas, n_i is the number of moles of the gas, n_total is the total number of moles of all gases in the mixture, and P_total is the total pressure of the mixture. This formula allows us to calculate the partial pressure of each gas in a mixture, given the total pressure and the composition of the mixture.
| Gas | Number of Moles | Partial Pressure |
|---|---|---|
| Oxygen (O2) | 2 moles | 0.4 atm |
| Nitrogen (N2) | 4 moles | 0.8 atm |
| Carbon Dioxide (CO2) | 1 mole | 0.2 atm |
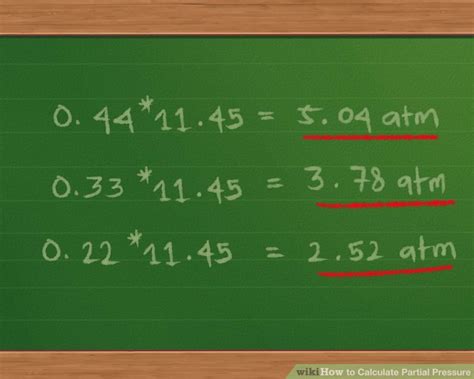
Methods to Calculate Partial Pressure
There are several methods to calculate partial pressure, each with its own advantages and limitations. Here are five common methods:
1. Dalton’s Law Method
This method is based on Dalton’s Law, which states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of each individual gas. The formula to calculate partial pressure using this method is: P_i = (n_i / n_total) * P_total.
2. Ideal Gas Law Method
The Ideal Gas Law provides another method to calculate partial pressure. The Ideal Gas Law states that PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature. By rearranging this equation, we can calculate the partial pressure of a gas: P_i = (n_i / V) * RT.
3. Mole Fraction Method
The mole fraction method is a simple and convenient way to calculate partial pressure. The mole fraction of a gas is defined as the number of moles of the gas divided by the total number of moles of all gases in the mixture. The partial pressure of a gas can be calculated using the formula: P_i = X_i * P_total, where X_i is the mole fraction of the gas.
4. Henry’s Law Method
Henry’s Law provides a method to calculate partial pressure of a gas in a liquid solution. The law states that the partial pressure of a gas in a liquid solution is directly proportional to the concentration of the gas in the solution. The formula to calculate partial pressure using this method is: P_i = k * C_i, where k is the Henry’s Law constant and C_i is the concentration of the gas in the solution.
5. Graham’s Law Method
Graham’s Law provides a method to calculate partial pressure of a gas based on its rate of diffusion. The law states that the rate of diffusion of a gas is inversely proportional to the square root of its molecular weight. The formula to calculate partial pressure using this method is: P_i = (R_i / R_total) * P_total, where R_i is the rate of diffusion of the gas and R_total is the total rate of diffusion of all gases in the mixture.
Applications and Implications
Calculating partial pressure has numerous applications in various fields, including chemistry, physics, engineering, and environmental science. For example, in scuba diving, the partial pressure of oxygen and nitrogen is crucial in determining the safe depth and time limits for divers. In atmospheric science, the partial pressure of greenhouse gases, such as carbon dioxide and methane, is essential in understanding climate change and its implications.
Real-World Examples
Let’s consider a few real-world examples to illustrate the importance of partial pressure calculations. In a scuba diving scenario, the partial pressure of oxygen and nitrogen can be calculated using Dalton’s Law. For instance, if the total pressure at a depth of 30 meters is 4 atm, and the composition of the air is 21% oxygen and 79% nitrogen, we can calculate the partial pressure of oxygen and nitrogen using the formula: P_i = (n_i / n_total) * P_total.
| Gas | Number of Moles | Partial Pressure |
|---|---|---|
| Oxygen (O2) | 0.21 moles | 0.84 atm |
| Nitrogen (N2) | 0.79 moles | 3.16 atm |
Conclusion
In conclusion, calculating partial pressure is a critical concept in understanding the behavior of gases. By mastering the different methods to calculate partial pressure, we can better appreciate the complexity of gas behavior and make more accurate predictions and calculations. Whether in chemistry, physics, engineering, or environmental science, the ability to calculate partial pressure is essential in a wide range of applications. As we continue to explore and understand the world around us, the importance of partial pressure calculations will only continue to grow.
What is partial pressure, and why is it important?
+Partial pressure refers to the pressure exerted by a single component of a mixture of gases. It is crucial in understanding the behavior of gases and has numerous applications in various fields, including chemistry, physics, engineering, and environmental science.
What are the different methods to calculate partial pressure?
+There are several methods to calculate partial pressure, including Dalton’s Law, the Ideal Gas Law, the mole fraction method, Henry’s Law, and Graham’s Law. Each method has its own advantages and limitations, and the choice of method depends on the specific problem and the available data.
What are some real-world applications of partial pressure calculations?
+Partial pressure calculations have numerous applications in various fields, including scuba diving, atmospheric science, engineering, and environmental science. For example, in scuba diving, the partial pressure of oxygen and nitrogen is crucial in determining the safe depth and time limits for divers. In atmospheric science, the partial pressure of greenhouse gases, such as carbon dioxide and methane, is essential in understanding climate change and its implications.