Calculating concentration is a fundamental concept in chemistry, biology, and other scientific fields. It refers to the amount of substance (solute) present in a given quantity of a mixture or solution. There are several ways to calculate concentration, each with its own application and relevance. Understanding these methods is crucial for accurate measurements and analysis in various scientific and industrial contexts. In this article, we will explore five primary methods for calculating concentration, including their formulas, applications, and examples.
Key Points
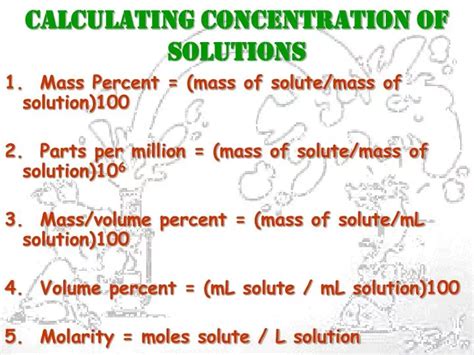
- Molarity is a common method for calculating concentration, defined as the number of moles of solute per liter of solution.
- Molality is used when the mass of the solvent is known, expressed as the number of moles of solute per kilogram of solvent.
- Mass percentage is a straightforward method, representing the mass of the solute as a percentage of the total mass of the solution.
- Volume percentage is similar to mass percentage but is based on volumes rather than masses, useful for mixtures of liquids.
- Parts per million (ppm) or parts per billion (ppb) are used for very dilute solutions, representing the ratio of the mass of the solute to the mass of the solution in parts per million or billion.
Molarity
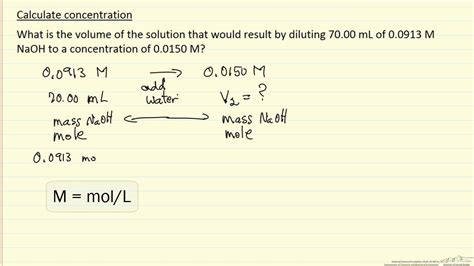
Molarity (M) is one of the most commonly used units of concentration. It is defined as the number of moles of solute per liter of solution. The formula for calculating molarity is M = moles of solute / liters of solution. For example, if you have 2 moles of sodium chloride (NaCl) dissolved in enough water to make 1 liter of solution, the molarity of the solution is 2 M. Molarity is particularly useful in chemical reactions and titrations, where the amount of substance reacting is critical.
Calculating Molarity
To calculate molarity, you first need to know the number of moles of the solute. This can be calculated from the mass of the solute using its molar mass. For instance, the molar mass of NaCl is approximately 58.44 g/mol. If you have 100 grams of NaCl, you can calculate the number of moles as follows: moles = mass of NaCl / molar mass of NaCl = 100 g / 58.44 g/mol = 1.71 mol. If this amount of NaCl is dissolved in water to make 1 liter of solution, the molarity would be 1.71 M.
Molality
Molality (m) is another method for expressing concentration, defined as the number of moles of solute per kilogram of solvent. The formula for molality is m = moles of solute / kilograms of solvent. Molality is useful when the mass of the solvent is known, and it is not affected by the volume of the solution, which can change with temperature. This makes molality particularly useful in applications where temperature may vary.
Calculating Molality
Calculating molality requires knowing the mass of the solvent. For example, if you have 1 mole of sugar (C6H12O6, molar mass = 180.16 g/mol) dissolved in 1 kg of water, the molality of the sugar solution is 1 m. This method is particularly useful in contexts where the solvent’s mass is easier to measure accurately than the solution’s volume.
Mass Percentage
Mass percentage is a simple and straightforward method for expressing concentration. It is the mass of the solute divided by the total mass of the solution, multiplied by 100%. The formula is: mass percentage = (mass of solute / total mass of solution) * 100%. This method is useful for mixtures where the masses of the components are known, and it provides a quick way to express concentration in a percentage form.
Example of Mass Percentage
For instance, if you have 30 grams of NaCl dissolved in water to make a total solution mass of 300 grams, the mass percentage of NaCl can be calculated as follows: mass percentage = (30 g / 300 g) * 100% = 10%. This means the solution is 10% NaCl by mass.
Volume Percentage
Volume percentage is similar to mass percentage but is based on volumes instead of masses. It is defined as the volume of the solute divided by the total volume of the solution, multiplied by 100%. The formula is: volume percentage = (volume of solute / total volume of solution) * 100%. This method is particularly useful for mixtures of liquids where volumes are more relevant than masses.
Calculating Volume Percentage
For example, if you have 200 mL of ethanol mixed with enough water to make 1 liter (1000 mL) of solution, the volume percentage of ethanol is calculated as: volume percentage = (200 mL / 1000 mL) * 100% = 20%. This indicates that the solution is 20% ethanol by volume.
Parts Per Million (PPM) and Parts Per Billion (PPB)
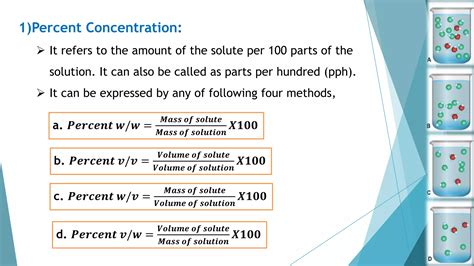
For very dilute solutions, concentration is often expressed in parts per million (ppm) or parts per billion (ppb). These units represent the ratio of the mass of the solute to the mass of the solution in parts per million or billion. The formulas are: ppm = (mass of solute in mg / mass of solution in kg) and ppb = (mass of solute in μg / mass of solution in kg). These methods are crucial in environmental science, where trace amounts of substances can have significant impacts.
Example of PPM and PPB
For instance, if a lake has 0.5 mg of lead per kilogram of water, the concentration of lead in ppm is 0.5 ppm. If the concentration is much lower, say 0.5 μg of lead per kilogram of water, then the concentration would be 0.5 ppb. These units allow for the precise measurement of very small concentrations, which is essential for assessing and managing environmental pollutants.
| Method | Formula | Units |
|---|---|---|
| Molarity | M = moles of solute / liters of solution | M (moles/L) |
| Molality | m = moles of solute / kilograms of solvent | m (moles/kg) |
| Mass Percentage | mass percentage = (mass of solute / total mass of solution) * 100% | % |
| Volume Percentage | volume percentage = (volume of solute / total volume of solution) * 100% | % |
| PPM/PPB | ppm = (mass of solute in mg / mass of solution in kg), ppb = (mass of solute in μg / mass of solution in kg) | ppm or ppb |
What is the primary difference between molarity and molality?
+Molarity is defined as the number of moles of solute per liter of solution, while molality is defined as the number of moles of solute per kilogram of solvent. This difference makes molarity dependent on the volume of the solution, which can change with temperature, whereas molality is independent of solution volume and thus is not affected by temperature changes.
When would you use parts per million (ppm) or parts per billion (ppb)?
+PPM and PPB are used for expressing very low concentrations of substances in a solution, typically in environmental or health contexts where even trace amounts can be significant. For example, measuring the concentration of pollutants in water or air often involves these units.
How does volume percentage differ from mass percentage?
+Volume percentage is based on the volumes of the solute and the solution, whereas mass percentage is based on their masses. This distinction makes volume percentage more suitable for mixtures of liquids, where volumes are easily measurable and relevant, while mass percentage is useful when the masses of the components are known and relevant.
In conclusion, calculating concentration is a fundamental aspect of various scientific disciplines, with different methods suited to different contexts and requirements. Understanding the principles behind molarity, molality, mass percentage, volume percentage, and parts per million/billion is essential for accurate measurements and analysis. By choosing the appropriate method based on the specific needs of the experiment or application, scientists and professionals can ensure precision and reliability in their work.