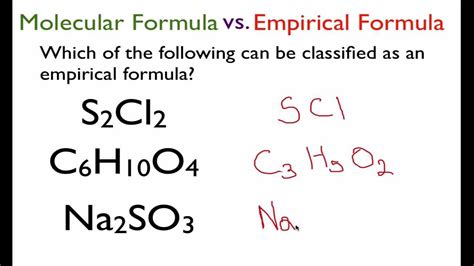
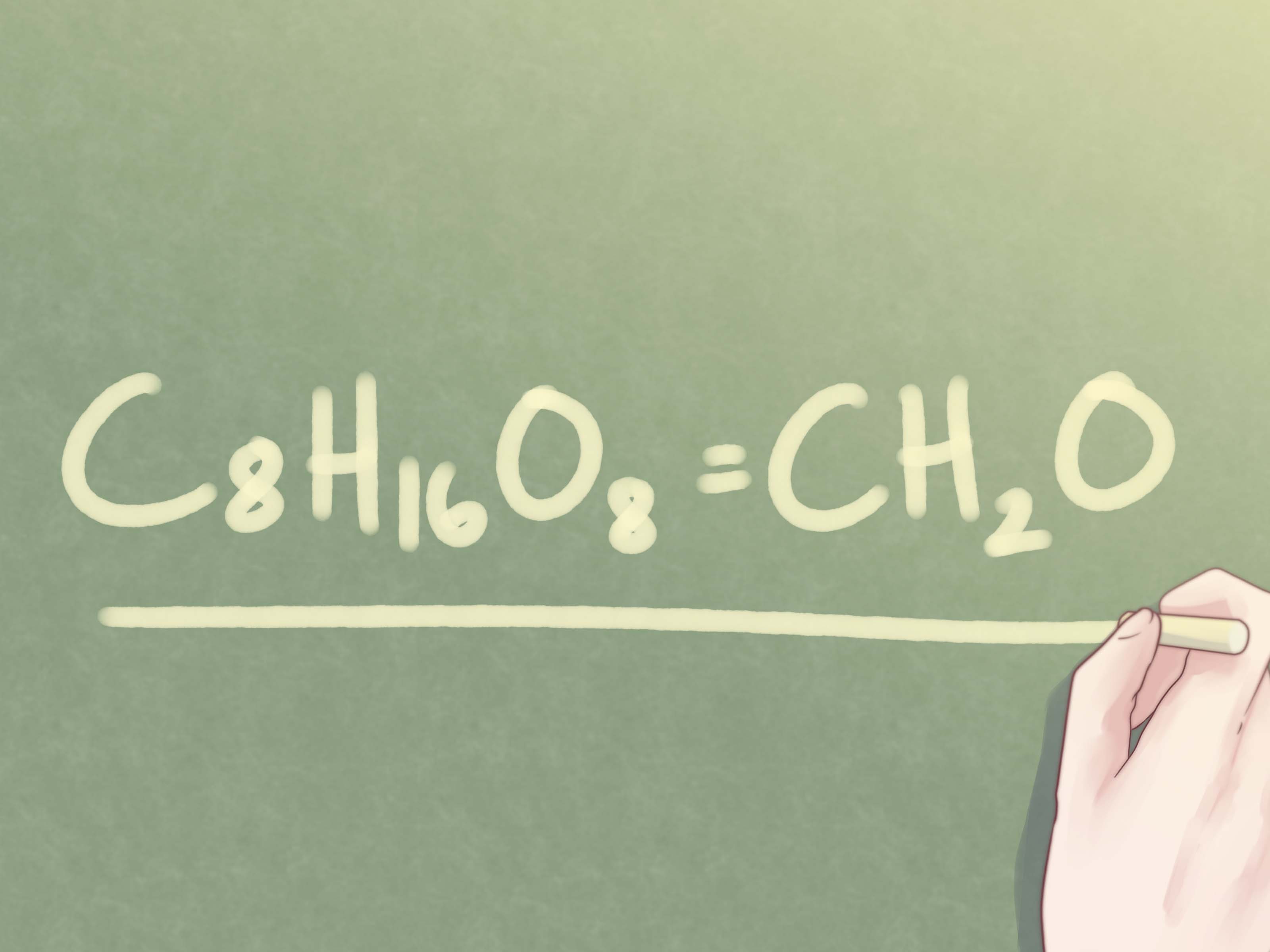Understanding the empirical formula of a compound is a fundamental aspect of chemistry, as it provides insight into the simplest whole-number ratio of atoms of each element present in the compound. The empirical formula is a crucial step in determining the molecular formula, which gives the actual number of atoms of each element in a molecule. In this article, we will delve into five methods to determine the empirical formula of a compound, highlighting the importance of empirical formulas in chemistry and the practical applications of these methods.
Key Points
- The empirical formula is the simplest whole-number ratio of atoms of each element in a compound.
- Percentage composition, combustion analysis, and mass spectrometry are common methods for determining empirical formulas.
- Understanding empirical formulas is essential for determining molecular formulas and understanding chemical reactions.
- Empirical formulas have practical applications in fields like pharmacology and materials science.
- Calculating empirical formulas involves converting percentages to masses, dividing by atomic masses, and finding the simplest whole-number ratio.
Introduction to Empirical Formulas
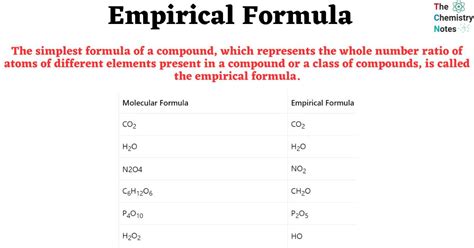
Empirical formulas are a basic representation of a compound’s composition, providing the simplest ratio of atoms of each element present. This is different from the molecular formula, which gives the actual number of atoms of each element in a single molecule of the compound. The empirical formula is an essential step in identifying a compound and understanding its properties and reactions.
Method 1: Percentage Composition
One of the most common methods for determining the empirical formula is through percentage composition. This involves knowing the percentage by mass of each element in the compound. To calculate the empirical formula, one converts the percentage of each element into grams, assuming a 100-gram sample of the compound. Then, by dividing the mass of each element by its atomic mass, one can find the number of moles of each element. Finally, by dividing each of these mole values by the smallest number of moles, one can determine the simplest whole-number ratio of atoms of each element, which is the empirical formula.
| Element | Percentage Composition | Atomic Mass |
|---|---|---|
| Carbon (C) | 40% | 12.01 g/mol |
| Hydrogen (H) | 6.67% | 1.008 g/mol |
| Oxygen (O) | 53.33% | 16.00 g/mol |
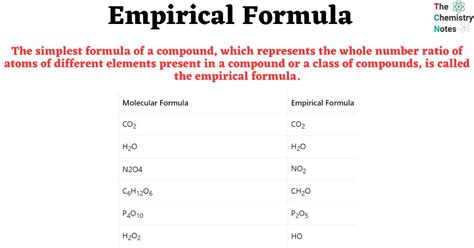
Method 2: Combustion Analysis
Combustion analysis is another method used to determine the empirical formula, especially for organic compounds. This method involves burning a sample of the compound in oxygen and measuring the amount of carbon dioxide and water produced. From these measurements, one can calculate the mass of carbon and hydrogen in the original sample. If other elements are present, additional tests may be required. Knowing the masses of these elements, one can proceed similarly to the percentage composition method to find the empirical formula.
Method 3: Mass Spectrometry
Mass spectrometry can also be used to determine the empirical formula by analyzing the mass-to-charge ratio of ions produced from the compound. This method can provide the molecular weight of the compound and, in some cases, the molecular formula directly. However, if the molecular formula is not directly obtainable, the empirical formula can still be determined by analyzing the fragmentation patterns and knowing the atomic masses of the elements involved.
Method 4: Chemical Analysis
Chemical analysis involves various chemical reactions and tests to determine the composition of a compound. For example, gravimetric analysis involves precipitating ions of a particular element and measuring the mass of the precipitate to determine the amount of that element in the compound. Volumetric analysis involves titrating a solution of the compound against a standard solution to determine the amount of a specific element or group. These methods can provide the percentage composition or the mass of elements in the compound, which can then be used to calculate the empirical formula.
Method 5: Spectroscopic Methods
Spectroscopic methods, such as nuclear magnetic resonance (NMR) spectroscopy and infrared (IR) spectroscopy, can provide detailed information about the molecular structure of a compound. While these methods do not directly give the empirical formula, they can provide information about the types of bonds and the ratio of certain elements, which can be used in conjunction with other methods to determine the empirical formula.
What is the main difference between the empirical and molecular formula?
+The empirical formula gives the simplest whole-number ratio of atoms of each element in a compound, while the molecular formula gives the actual number of atoms of each element in a molecule of the compound.
Why is determining the empirical formula important?
+Determining the empirical formula is important because it provides a basis for understanding the properties and reactions of a compound, and it is a necessary step in determining the molecular formula.
What are some common methods for determining the empirical formula?
+Common methods include percentage composition, combustion analysis, mass spectrometry, chemical analysis, and spectroscopic methods.
In conclusion, determining the empirical formula is a critical step in understanding the composition and properties of a compound. By using methods such as percentage composition, combustion analysis, mass spectrometry, chemical analysis, and spectroscopic methods, chemists can determine the empirical formula and lay the groundwork for further analysis and application. The empirical formula serves as a foundational piece of information, connecting the composition of a compound to its molecular structure and properties, and thus is a vital tool in various fields of chemistry and beyond.