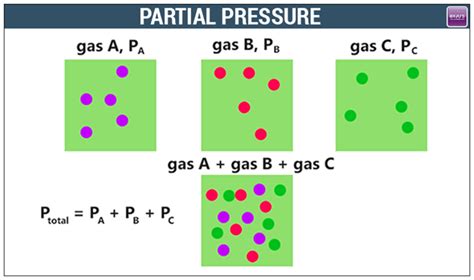
Determining partial pressure is a crucial concept in chemistry and physics, particularly in the study of gases. Partial pressure refers to the pressure exerted by a single component of a mixture of gases. It is a fundamental principle in understanding how gases interact and behave in various environments. There are several methods to determine partial pressure, each with its own set of applications and advantages. In this article, we will explore five key ways to determine partial pressure, highlighting their theoretical foundations, practical applications, and the contexts in which they are most appropriately used.
Key Points
- Understanding the concept of partial pressure and its significance in gas mixtures
- Dalton's Law of Partial Pressures as a foundational principle
- Calculating partial pressure using mole fractions and total pressure
- Experimental methods for determining partial pressure, including manometry and gas chromatography
- Applications of partial pressure in respiratory physiology and environmental science
1. Dalton’s Law of Partial Pressures
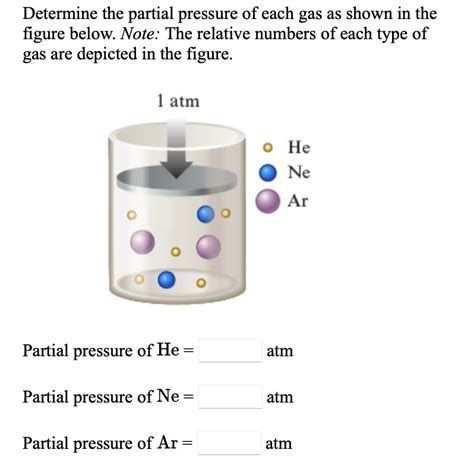
Dalton’s Law of Partial Pressures states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of each individual gas in the mixture. This law provides a fundamental method for calculating partial pressure. According to Dalton’s Law, the partial pressure of a gas can be calculated if the total pressure of the mixture and the mole fraction of the gas are known. The formula derived from Dalton’s Law for calculating partial pressure (Pi) of a gas is Pi = Ptotal * Xi, where Ptotal is the total pressure of the gas mixture and Xi is the mole fraction of the gas.
Calculating Mole Fraction
To apply Dalton’s Law, one must first calculate the mole fraction of the gas of interest. The mole fraction (Xi) of a gas in a mixture is defined as the number of moles of that gas divided by the total number of moles of all gases in the mixture. For example, in a mixture containing 2 moles of oxygen, 3 moles of nitrogen, and 1 mole of carbon dioxide, the mole fraction of oxygen would be 2 / (2 + 3 + 1) = 2⁄6 = 1⁄3. If the total pressure of this mixture is 1 atm, the partial pressure of oxygen would be 1 atm * (1⁄3) = 1⁄3 atm.
2. Manometry
Manometry is a direct experimental method used to measure pressure, including partial pressure. In the context of partial pressure determination, manometry involves measuring the pressure exerted by a gas in a sealed system. This can be achieved using a manometer, which is essentially a U-shaped tube filled with a liquid, where the difference in liquid levels in the two arms of the tube is proportional to the pressure difference. By isolating a gas in one arm of the manometer and knowing the atmospheric pressure (or the pressure in the other arm), one can directly measure the partial pressure of the gas.
Practical Considerations
Practical considerations when using manometry include ensuring that the system is sealed to prevent gas escape or ingress, selecting an appropriate liquid for the manometer that does not react with the gas being measured, and accounting for factors like temperature and humidity that can affect the accuracy of the measurements.
3. Gas Chromatography
Gas chromatography (GC) is a sophisticated analytical technique that can separate and analyze the components of a gas mixture. By separating the gases based on their boiling points, affinity for the stationary phase, or other properties, GC can provide the composition of the gas mixture. Knowing the composition (i.e., the mole fraction of each gas) and the total pressure of the mixture allows for the calculation of the partial pressure of each gas component using Dalton’s Law.
Applications of Gas Chromatography
Gas chromatography has a wide range of applications, from environmental monitoring (e.g., analyzing air quality) to industrial process control (e.g., monitoring gas streams in chemical plants). Its ability to provide detailed compositional analysis of gas mixtures makes it an invaluable tool for determining partial pressures in complex systems.
4. Vapor Pressure Measurement
For gases that are in equilibrium with their liquid phase, such as water vapor in air, the partial pressure can be determined by measuring the vapor pressure of the liquid. Vapor pressure is a characteristic property of a liquid at a given temperature, representing the pressure exerted by the vapor in equilibrium with the liquid. Techniques like the use of a vapor pressure osmometer can measure vapor pressure, from which the partial pressure of the vapor in a gas mixture can be inferred.
Vapor Pressure and Humidity
In the context of atmospheric science, measuring the vapor pressure of water is crucial for understanding humidity levels and related meteorological phenomena. The partial pressure of water vapor in the atmosphere is a key factor in determining weather patterns, including precipitation and cloud formation.
5. Membrane Separation

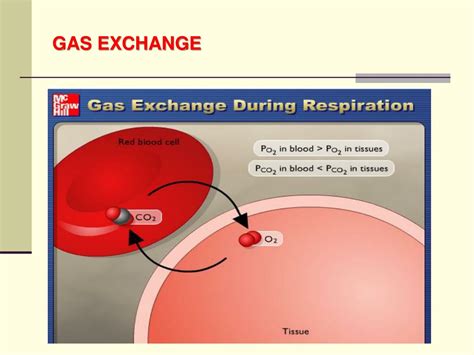
Membrane separation techniques can also be used to determine partial pressure, particularly in biological systems or for gases with specific membrane permeabilities. By selectively allowing certain gases to pass through a membrane, one can isolate and measure the pressure of specific gases within a mixture. This method is especially useful in medical applications, such as measuring the partial pressures of oxygen and carbon dioxide in blood.
Biological Applications
In respiratory physiology, understanding the partial pressures of oxygen and carbon dioxide is vital for assessing gas exchange in the lungs and overall respiratory function. Membrane separation techniques, combined with sensors or other analytical methods, can provide real-time measurements of these critical parameters.
What is the significance of partial pressure in gas mixtures?
+Partial pressure is significant because it determines the concentration and behavior of gases in mixtures, influencing chemical reactions, physical properties, and biological processes.
How does Dalton's Law relate to partial pressure?
+Dalton's Law states that the total pressure of a gas mixture is the sum of the partial pressures of its components, providing a fundamental method for calculating partial pressure.
What are some common methods for measuring partial pressure?
+Common methods include using manometry, gas chromatography, vapor pressure measurement, and membrane separation techniques, each with its specific applications and advantages.
In conclusion, determining partial pressure is a multifaceted task that can be approached through various theoretical and experimental methods. Understanding the principles behind these methods, including Dalton’s Law, and being familiar with experimental techniques like manometry and gas chromatography, is essential for accurately determining partial pressures in different contexts. The applications of partial pressure determination are vast, ranging from industrial process control and environmental monitoring to medical diagnostics and respiratory physiology, underscoring the importance of this concept in both scientific research and practical applications.