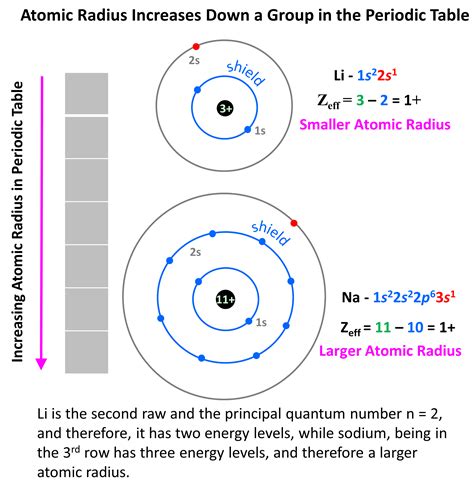
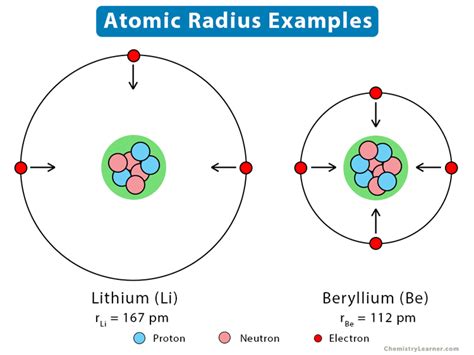
The atomic radius is a fundamental concept in chemistry, referring to the distance from the nucleus of an atom to the outermost electron in its orbit. Understanding how to find the atomic radius is crucial for various applications in physics, chemistry, and materials science. There are several methods to determine the atomic radius, each with its own set of assumptions and limitations. In this article, we will explore five ways to find the atomic radius, discussing the principles behind each method, their applications, and the challenges associated with them.
Key Points
- The atomic radius can be determined through experimental methods such as X-ray diffraction and electron diffraction.
- Theoretical models, including the Bohr model and quantum mechanical calculations, provide another avenue for estimating atomic radii.
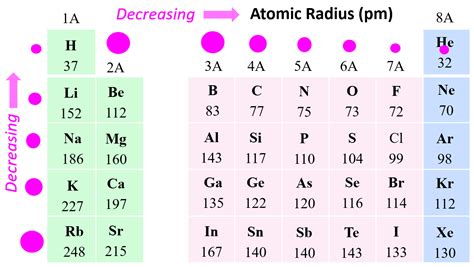
- Empirical formulas, like the one derived from the periodic table trends, offer a simplified approach to predicting atomic radii.
- Comparing atomic radii across different elements and their compounds can reveal valuable insights into chemical bonding and reactivity.
- The choice of method depends on the specific requirements of the study, including the desired level of accuracy and the availability of experimental data.
X-Ray Diffraction Method
X-ray diffraction is a powerful technique for determining the structure of crystalline materials, including the atomic radius of elements within these structures. By analyzing how X-rays scatter off the electrons in a crystal lattice, researchers can infer the distances between atoms and, consequently, their radii. This method is particularly useful for elements that form crystals, as it provides direct information about the atomic arrangement in the solid state. The X-ray diffraction technique relies on the principle that the scattering of X-rays by the electrons in an atom is related to the electron density distribution around the nucleus.
Principle of X-Ray Diffraction
The principle behind X-ray diffraction is based on Bragg’s law, which states that the diffraction of X-rays by a crystal lattice occurs at specific angles where the path difference between X-rays scattered by adjacent planes of atoms is an integer number of wavelengths. By measuring these angles and knowing the wavelength of the X-rays, one can calculate the interplanar distances and, from these, infer the atomic radius. The accuracy of this method depends on the quality of the crystal, the wavelength of the X-rays used, and the precision of the angle measurements.
| Technique | Description | Advantages |
|---|---|---|
| X-Ray Diffraction | Determines atomic radius through crystal structure analysis | High accuracy for crystalline materials |
| Electron Diffraction | Similar to X-ray diffraction but uses electrons | Suitable for materials that do not form good crystals |
| Bohr Model | Theoretical model for estimating atomic radius | Simple and intuitive for understanding basic principles |
| Quantum Mechanical Calculations | Computational method for precise atomic radius calculation | High accuracy but computationally intensive |
| Empirical Formulas | Simplified approach based on periodic table trends | Easy to apply but less accurate than other methods |
Electron Diffraction Method

Electron diffraction is another experimental technique used to determine the structure of materials, including the atomic radius of elements. This method is particularly useful for substances that do not form good crystals, as it can provide structural information from amorphous or powdery samples. Electron diffraction works on a similar principle to X-ray diffraction but uses a beam of electrons instead of X-rays. The electrons are scattered by the atoms in the sample, and by analyzing the diffraction pattern, researchers can infer the atomic arrangement and, hence, the atomic radius.
Applications of Electron Diffraction
Electron diffraction has found applications in materials science and chemistry, especially in the study of nanostructures and surface science. It provides a means to investigate the structure of materials at the atomic level, which is crucial for understanding their properties and behavior. The technique is also valuable for studying the structure of biological molecules and complexes, offering insights into their function and interaction at the molecular level.
Theoretical Models for Atomic Radius
Theoretical models, such as the Bohr model and quantum mechanical calculations, offer a way to estimate the atomic radius without the need for experimental data. The Bohr model, although simplistic, provides a basic understanding of how electrons are arranged around the nucleus and can be used to estimate the atomic radius of hydrogen-like atoms. Quantum mechanical calculations, on the other hand, provide a more accurate and detailed description of the atomic structure, allowing for the precise calculation of atomic radii for a wide range of elements.
Quantum Mechanical Approach
Quantum mechanical calculations involve solving the Schrödinger equation for the atom, which describes the behavior of electrons in terms of wave functions. These calculations can provide detailed information about the electron density distribution around the nucleus, from which the atomic radius can be inferred. While highly accurate, these calculations are computationally intensive and require significant resources, especially for larger atoms and molecules.
In conclusion, determining the atomic radius is a complex task that can be approached through various experimental and theoretical methods. Each method has its strengths and limitations, and the choice of which to use depends on the specific context and the desired level of accuracy. By understanding the principles behind these methods and their applications, researchers can gain valuable insights into the structure and properties of atoms and molecules, which is essential for advancing our knowledge in physics, chemistry, and materials science.
What is the most accurate method for determining the atomic radius?
+Quantum mechanical calculations are generally considered the most accurate method for determining the atomic radius, as they provide a detailed description of the electron density distribution around the nucleus.
Can the atomic radius be determined for all elements?
+While the atomic radius can be determined for most elements, there are challenges associated with certain elements, particularly those that do not form stable crystals or have complex electronic structures.
How does the atomic radius relate to the chemical properties of an element?
+The atomic radius is closely related to the chemical properties of an element, including its reactivity, electronegativity, and ability to form compounds with other elements. Generally, elements with smaller atomic radii tend to be more reactive and have higher electronegativities.