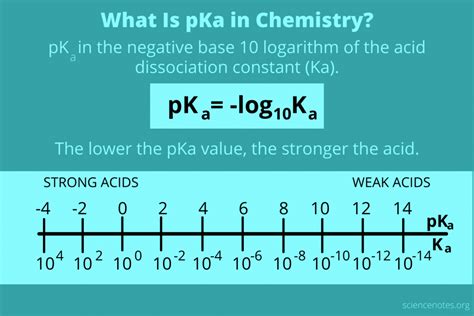
Understanding the relationship between pKa and Ka is crucial in chemistry, particularly in the context of acid-base reactions. The pKa of an acid is a measure of its strength, with lower pKa values indicating stronger acids. However, when working with chemical equations or solving problems, it's often necessary to find the Ka (acid dissociation constant) from the pKa. This process is straightforward and involves a simple mathematical relationship.
Understanding pKa and Ka
The pKa of an acid is defined as the negative logarithm of the acid dissociation constant (Ka). Mathematically, this is expressed as pKa = -log(Ka). This relationship allows for the easy conversion between pKa and Ka, providing a comprehensive understanding of an acid’s properties.
Converting pKa to Ka
To find the Ka from the pKa, one uses the formula Ka = 10^(-pKa). This formula is derived directly from the definition of pKa. By applying this formula, chemists can quickly determine the Ka of an acid, given its pKa value. For instance, if the pKa of an acid is 4.5, its Ka would be calculated as Ka = 10^(-4.5), resulting in a specific value that quantifies the acid’s dissociation properties.
| pKa Value | Calculated Ka |
|---|---|
| 4.5 | 3.16227766e-5 |
| 5.0 | 1e-5 |
| 3.0 | 0.001 |

Practical Applications

Understanding how to find Ka from pKa has numerous practical applications in chemistry, including the prediction of acid-base reaction outcomes, the design of buffers, and the analysis of chemical equilibria. By knowing the Ka of an acid, chemists can better understand its behavior in various chemical contexts, facilitating the synthesis of new compounds and the optimization of existing processes.
Calculation Example
Consider an acid with a pKa of 2.8. To find its Ka, one would apply the formula Ka = 10^(-2.8). Calculating this gives a Ka of approximately 1.58 x 10^(-3), indicating the acid’s strength and its tendency to donate a proton in solution. This kind of calculation is fundamental in assessing the acid’s potential interactions with bases or other acids in chemical reactions.
Key Points
- The relationship between pKa and Ka is given by pKa = -log(Ka), allowing for the conversion between the two.
- To find Ka from pKa, the formula Ka = 10^(-pKa) is used.
- Small changes in pKa result in significant changes in Ka due to the logarithmic scale of pKa.
- Understanding Ka is crucial for predicting acid-base reaction outcomes and designing chemical processes.
- The calculation of Ka from pKa is a fundamental skill in chemistry, applicable to various chemical contexts.
Mastering the conversion between pKa and Ka is essential for chemists and chemistry students, as it provides a deeper understanding of acid-base chemistry and facilitates the analysis and prediction of chemical reactions. By applying the simple yet powerful relationship between these two constants, one can unlock a wealth of information about the properties and behaviors of acids in chemical systems.
What is the formula to find Ka from pKa?
+The formula to find Ka from pKa is Ka = 10^(-pKa).
Why is understanding Ka important in chemistry?
+Understanding Ka is important because it helps in predicting the strength of an acid, designing buffers, and analyzing chemical equilibria, which are crucial in various chemical processes and applications.
How does the logarithmic scale of pKa affect the calculation of Ka?
+The logarithmic scale of pKa means that small changes in pKa values result in significant changes in Ka values, emphasizing the importance of precise pKa measurements for accurate calculations.