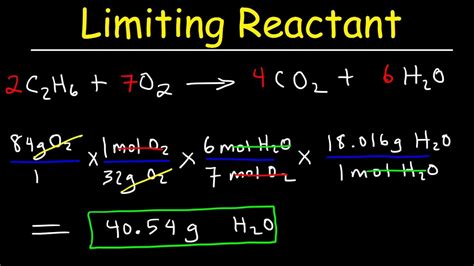
The concept of a limiting reactant is crucial in chemistry, particularly in stoichiometry, as it determines the maximum amount of product that can be formed in a chemical reaction. The limiting reactant is the reactant that is completely consumed first, thereby limiting the amount of product that can be formed. Finding the limiting reactant is essential for optimizing reaction conditions, predicting yields, and managing resources efficiently. Here, we will explore five ways to identify the limiting reactant in a chemical reaction.
Understanding the Basics of Limiting Reactants
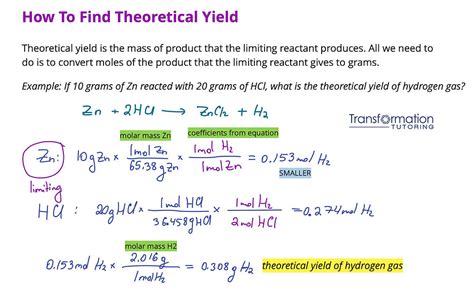
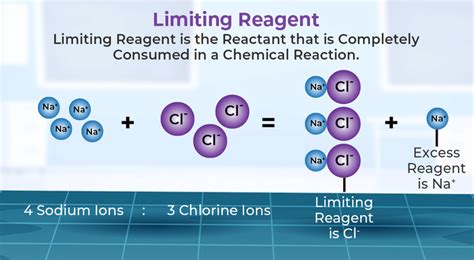
Before diving into the methods for finding the limiting reactant, it’s essential to understand the basic principles. In any chemical reaction, reactants are consumed to produce products. The stoichiometry of the reaction, as indicated by the balanced chemical equation, determines the molar ratios of reactants to products. When one reactant is completely consumed before the others, it becomes the limiting reactant. This reactant dictates the maximum amount of product that can be formed, regardless of the amounts of other reactants present.
Method 1: Comparison of Mole Ratios
One of the most straightforward methods to identify the limiting reactant is by comparing the mole ratios of the reactants provided to the mole ratios required by the balanced chemical equation. This method involves calculating the number of moles of each reactant available and then determining which reactant will be completely consumed first based on the stoichiometric ratios. For example, consider a reaction requiring 2 moles of A for every 3 moles of B. If you have 6 moles of A and 7 moles of B, A would be the limiting reactant because it would be completely consumed first, leaving some B unreacted.
| Reactant | Moles Available | Moles Required |
|---|---|---|
| A | 6 | 6 |
| B | 7 | 9 |
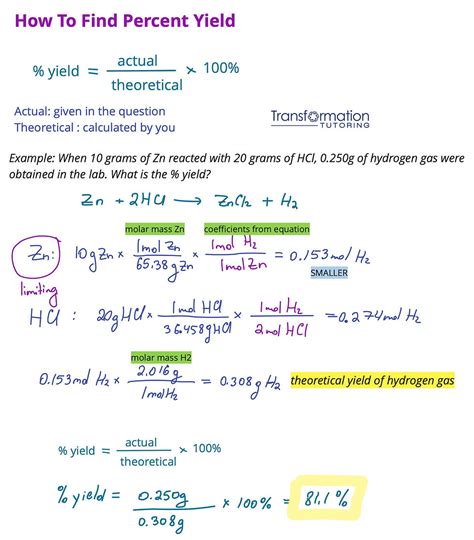
Calculating the Limiting Reactant through Mole-to-Mole Comparison
A more detailed approach to identifying the limiting reactant involves calculating how much of each reactant is needed to completely react with the other reactants. This method is particularly useful when dealing with complex reactions involving multiple reactants. By dividing the amount of each reactant available by the stoichiometric coefficient from the balanced equation, you can determine which reactant will be used up first.
Method 2: Using Conversion Factors
Conversion factors can be a powerful tool in identifying the limiting reactant, especially in reactions where the stoichiometry is complex. By setting up conversion factors from the given amounts of reactants to the amounts of products, one can determine which reactant limits the reaction. This method involves converting the given masses or volumes of reactants into moles and then using the stoichiometric ratios from the balanced equation to find out which reactant is limiting.
For instance, in the reaction 2A + 3B → C, if you have 100 grams of A and 150 grams of B, you first convert these masses into moles using their molar masses. Then, using the stoichiometric ratios, you calculate how much of one reactant is needed to react with the other completely, thus identifying the limiting reactant.
Key Points
- Identify the balanced chemical equation to determine stoichiometric ratios.
- Calculate the moles of each reactant available.
- Compare the mole ratios of reactants to the ratios required by the reaction.
- Use conversion factors to relate reactants to products based on stoichiometry.
- Determine the limiting reactant based on which is completely consumed first.
Practical Applications and Considerations
In practical scenarios, identifying the limiting reactant is crucial for maximizing the yield of the desired product and minimizing waste. This is particularly important in industrial processes where efficiency and cost-effectiveness are paramount. By understanding which reactant limits the reaction, chemists and engineers can optimize reaction conditions, such as temperature, pressure, and reactant ratios, to achieve the best possible outcomes.
Method 3: Graphical Method
A graphical method can also be employed to find the limiting reactant, especially in reactions involving two reactants. By plotting the amount of one reactant against the amount of the other, with the stoichiometric ratio as a reference line, one can visually identify which reactant is limiting. This method is intuitive and can be useful for quick assessments or educational purposes.
Advanced Considerations and Complex Reactions
In complex reactions or those involving multiple steps, identifying the limiting reactant can be more challenging. It may require breaking down the reaction into its constituent steps, analyzing the stoichiometry of each, and considering the kinetics of the reaction. Additionally, factors such as catalysts, inhibitors, or side reactions can influence which reactant is limiting, necessitating a comprehensive understanding of the reaction mechanism.
Method 4: Calculating Theoretical Yields
Another approach to identifying the limiting reactant involves calculating the theoretical yield of the product based on each reactant. The reactant that results in the lowest theoretical yield of the product is the limiting reactant. This method is particularly useful when the goal is to predict the maximum amount of product that can be formed under given conditions.
Method 5: Experimental Determination
Finally, the limiting reactant can be determined experimentally by conducting the reaction with varying amounts of each reactant and measuring the yield of the product. While this method is more time-consuming and resource-intensive, it can provide definitive evidence of which reactant is limiting under specific reaction conditions.
What is the significance of identifying the limiting reactant in a chemical reaction?
+Identifying the limiting reactant is crucial for optimizing reaction conditions, predicting yields, and managing resources efficiently. It helps in maximizing the amount of product formed and minimizing waste.
How does the stoichiometry of a reaction influence the identification of the limiting reactant?
+The stoichiometry of a reaction, as indicated by the balanced chemical equation, provides the molar ratios of reactants to products. These ratios are essential for determining which reactant is limiting based on the amounts available.
What are the practical implications of identifying the limiting reactant in industrial processes?
+In industrial processes, identifying the limiting reactant is vital for optimizing production, reducing costs, and ensuring environmental sustainability. It helps in the efficient use of resources, minimization of waste, and maximization of product yield.
In conclusion, identifying the limiting reactant is a fundamental aspect of chemical reactions, with significant implications for reaction optimization, yield prediction, and resource management. By understanding the five methods outlined here—comparison of mole ratios, using conversion factors, graphical method, calculating theoretical yields, and experimental determination—chemists and engineers can better design and execute chemical reactions to achieve desired outcomes efficiently and sustainably.