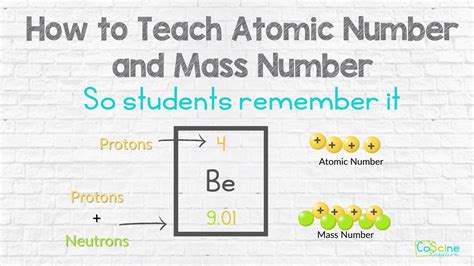
Protons, positively charged subatomic particles, reside in the nucleus of an atom, playing a crucial role in defining the chemical properties of elements. Finding protons, or more accurately, determining their number in an atom, is essential in chemistry and physics. The number of protons in an atom's nucleus determines the element of an atom, with each element having a unique number of protons in its atoms, known as the atomic number. Here, we'll explore five methods to find or determine the number of protons in an atom, each with its unique applications and significance in scientific research and education.
Key Points
- Determining the atomic number through periodic table analysis
- Using mass spectrometry for precise measurements
- Applying the principle of atomic number conservation in nuclear reactions
- Employing X-ray fluorescence for elemental analysis
- Utilizing particle accelerators for high-energy collision experiments
Understanding Protons and Their Role
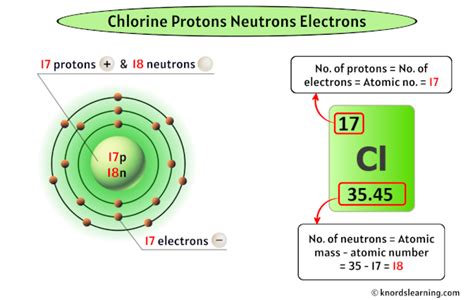
Protons, along with neutrons, constitute the nucleus of an atom, and the number of protons defines the chemical element. For instance, hydrogen has one proton, helium has two, and so on. The determination of protons is crucial for identifying elements, understanding chemical reactions, and predicting the properties of compounds. Each method of finding protons has its specific applications, ranging from basic chemical analysis to advanced nuclear physics research.
Method 1: Periodic Table Analysis
The periodic table is a powerful tool for determining the number of protons in an atom. By locating an element on the periodic table, one can directly find its atomic number, which is the number of protons in the nucleus of an atom of that element. This method is straightforward and widely used in educational settings to introduce students to the basics of chemistry. For example, to find the number of protons in a carbon atom, one would look at the periodic table and find that carbon has an atomic number of 6, meaning it has 6 protons.
Method 2: Mass Spectrometry
Mass spectrometry is a technique used to determine the mass-to-charge ratio of ions. It can be used to find the number of protons in an atom by analyzing the mass spectrum of a sample. This method is particularly useful for identifying and quantifying the elements present in a sample. By ionizing the sample and separating the ions based on their mass-to-charge ratio, mass spectrometry can provide precise information about the atomic and molecular composition of a substance, including the number of protons in each atom.
| Method | Description | Application |
|---|---|---|
| Mass Spectrometry | Determines mass-to-charge ratio of ions | Elemental analysis, isotopic identification |
| Periodic Table Analysis | Locates elements based on atomic number | Chemistry education, quick elemental identification |
| Nuclear Reactions | Conservation of atomic number in reactions | Nuclear physics research, radioactive decay studies |
| X-ray Fluorescence | Analyzes X-ray emission spectra | Material analysis, forensic science |
| Particle Accelerators | High-energy collisions to study subatomic particles | Particle physics research, discovery of new particles |

Method 3: Atomic Number Conservation in Nuclear Reactions
In nuclear reactions, the total number of protons (atomic number) is conserved. By analyzing the reactants and products of a nuclear reaction, one can determine the number of protons involved. This principle is fundamental in nuclear physics and is used to predict the outcomes of various nuclear processes, including radioactive decay and nuclear fission or fusion reactions.
Method 4: X-ray Fluorescence (XRF)
XRF is a non-destructive analytical technique used to determine the elemental composition of materials. When a sample is exposed to X-rays, the atoms in the sample emit characteristic X-rays, whose energies are specific to each element. By analyzing the X-ray emission spectrum, one can identify the elements present in the sample and, therefore, determine the number of protons in the atoms of those elements. XRF is widely used in material science, archaeology, and forensic science.
Method 5: Particle Accelerators
Particle accelerators accelerate charged particles, such as protons or electrons, to very high energies. By studying the collisions of these particles with target nuclei, scientists can gain insights into the structure of atoms and nuclei. Particle accelerators have been instrumental in the discovery of new subatomic particles and in understanding the strong nuclear force that holds protons and neutrons together in the nucleus.
In conclusion, determining the number of protons in an atom is crucial for understanding the properties of elements and their chemical behavior. From simple periodic table analysis to complex experiments using particle accelerators, each method provides valuable insights into the atomic structure and has its unique applications in science and technology.
What is the significance of determining the number of protons in an atom?
+Determining the number of protons in an atom is essential for identifying the element, understanding its chemical properties, and predicting its behavior in chemical reactions.
How does mass spectrometry help in finding the number of protons?
+Mass spectrometry helps by analyzing the mass-to-charge ratio of ions, allowing for the identification of elements based on their atomic mass and charge, which is directly related to the number of protons.
What are the applications of X-ray fluorescence in determining the number of protons?
+X-ray fluorescence is used in material analysis, forensic science, and archaeology to determine the elemental composition of samples, thereby identifying the elements present and their respective numbers of protons.