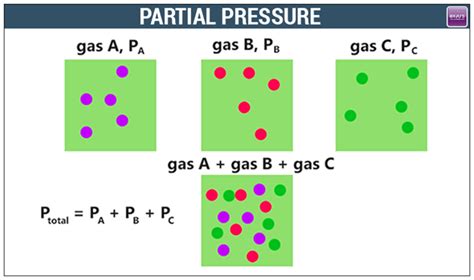
When dealing with gas mixtures, the concept of partial pressure is crucial for understanding the behavior and properties of each component. Partial pressure refers to the pressure exerted by a single component of a mixture of gases. It is a vital concept in chemistry and physics, especially in studies involving the ideal gas law, Dalton's law of partial pressures, and the behavior of gases in various conditions. Here, we explore five ways to find the partial pressure of a gas in a mixture, emphasizing the principles and formulas that underpin these methods.
Understanding Partial Pressure
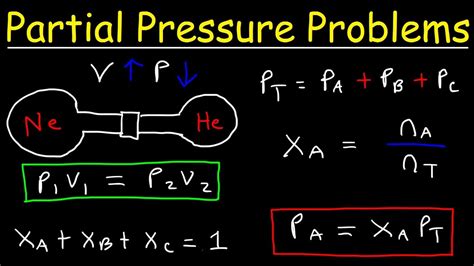
Before diving into the methods for finding partial pressure, it’s essential to grasp the fundamental principles. The partial pressure of a gas in a mixture is the pressure it would exert if it alone occupied the entire volume of the mixture at the same temperature. This concept is encapsulated in Dalton’s law of partial pressures, which states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of each individual gas. Mathematically, this can be expressed as P_total = P_1 + P_2 +… + P_n, where P_total is the total pressure of the mixture, and P_1, P_2,…, P_n are the partial pressures of the individual gases.
Method 1: Using Dalton’s Law of Partial Pressures
Dalton’s law provides a direct method to calculate the partial pressure of a gas if the total pressure and the partial pressures of the other gases are known. For a two-component gas mixture, if the total pressure (P_total) and the mole fraction (X) of one gas are known, the partial pressure (P) of that gas can be found using the formula P = X * P_total. This method is straightforward and relies on the accurate measurement or calculation of the mole fractions and the total pressure of the mixture.
| Gas Component | Mole Fraction (X) | Partial Pressure (P) |
|---|---|---|
| Oxygen (O2) | 0.21 | P_O2 = 0.21 * P_total |
| Nitrogen (N2) | 0.79 | P_N2 = 0.79 * P_total |
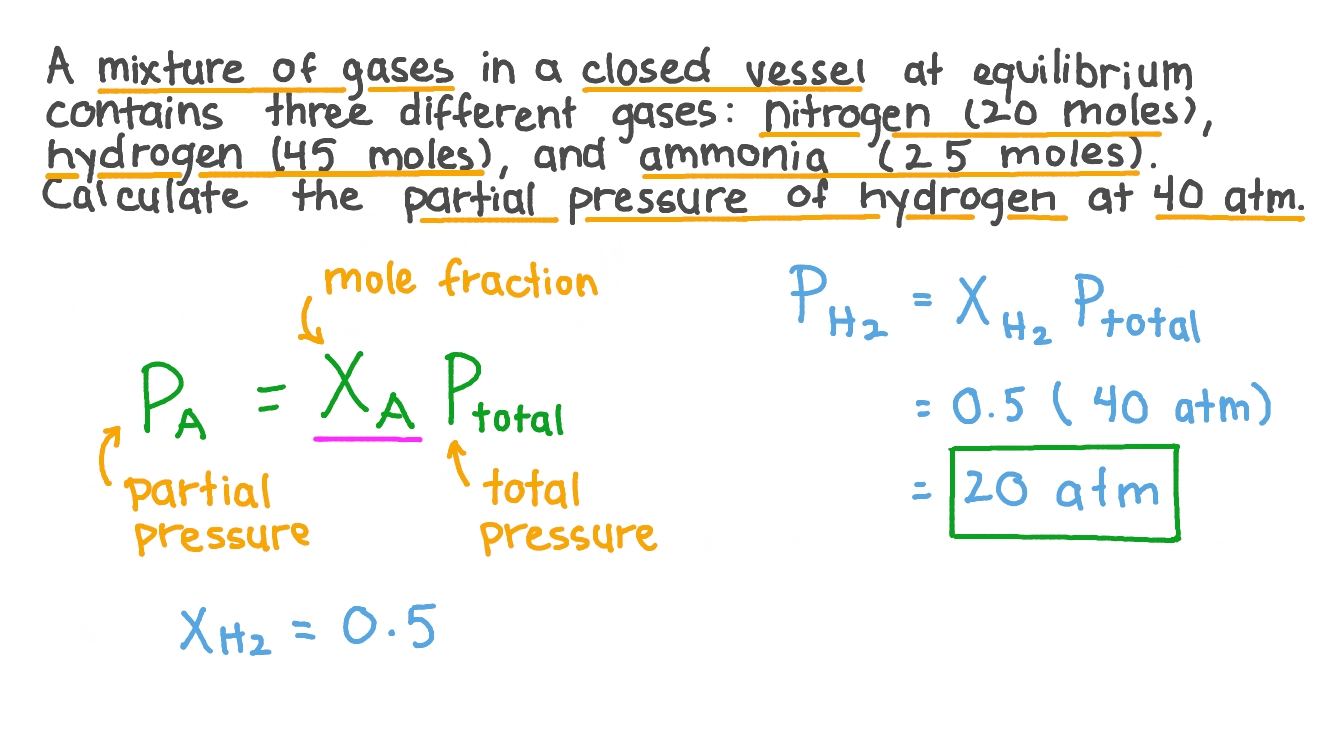
Calculating Partial Pressure from Mole Fractions
In scenarios where the mole fractions of the gases are known, and the total pressure of the mixture is given, calculating the partial pressure involves a simple multiplication. This method is particularly useful in laboratory settings where the composition of gas mixtures is controlled and known. The formula, as mentioned, is P = X * P_total, where P is the partial pressure of the gas, X is its mole fraction, and P_total is the total pressure of the mixture.
Method 2: From the Ideal Gas Law
The ideal gas law, PV = nRT, can also be used to derive the partial pressure of a gas in a mixture, given that the volume (V), the number of moles (n), the gas constant ®, and the temperature (T) are known for the specific gas and the mixture as a whole. By rearranging the ideal gas law to solve for pressure (P = nRT/V), and considering the number of moles and volume for the specific gas, one can calculate its partial pressure. This method is especially useful when dealing with gases at standard temperature and pressure (STP) conditions.
Practical Considerations for Partial Pressure Calculations
In practical scenarios, the calculation of partial pressure is often simplified by assuming ideal gas behavior. However, real gases exhibit non-ideal behavior, particularly under conditions far from STP. For precise calculations, especially in industrial or high-pressure applications, corrections for non-ideal behavior using equations like the Van der Waals equation may be necessary. These equations account for the attractive and repulsive forces between molecules, providing a more accurate representation of real gas behavior.
Method 3: Using Henry’s Law for Solutions
For gases dissolved in liquids, Henry’s law provides a method to estimate the partial pressure of the gas above the solution. Henry’s law states that the amount of a gas dissolved in a liquid is directly proportional to its partial pressure above the liquid. Mathematically, this is expressed as P = k * C, where P is the partial pressure of the gas, k is Henry’s law constant (which depends on the gas, the solvent, and the temperature), and C is the concentration of the gas in the liquid. This method is particularly relevant in studies involving the solubility of gases in blood or other biological fluids.
| Gas | Henry's Law Constant (k) | Concentration (C) | Partial Pressure (P) |
|---|---|---|---|
| Oxygen (O2) | 1.3 L*atm/mol | 0.01 mol/L | P_O2 = 1.3 * 0.01 |
Key Points
- The partial pressure of a gas in a mixture can be found using Dalton's law, the ideal gas law, Henry's law, or by analyzing the mole fractions and total pressure.
- Understanding the principles behind each method is crucial for accurate calculations and applications in various fields.
- Real gases may deviate from ideal behavior, necessitating corrections for non-ideal behavior in certain conditions.
- Henry's law is specifically useful for estimating the partial pressure of gases dissolved in liquids.
- Accurate measurements of total pressure, mole fractions, and concentrations are critical for reliable calculations of partial pressure.
Method 4: Analysis of Mole Fractions and Total Pressure
This method involves directly analyzing the composition of the gas mixture in terms of mole fractions and the total pressure to calculate the partial pressure of each component. It’s a straightforward approach when the mole fractions are known or can be determined through chemical analysis. The calculation simply involves multiplying the mole fraction of the gas by the total pressure of the mixture.
Method 5: Experimental Measurement
In some cases, especially in laboratory or industrial settings, the partial pressure of a gas can be measured directly using specialized equipment such as pressure sensors or gas analyzers. This method provides a direct and accurate measurement of the partial pressure without the need for calculations based on theoretical models. However, it requires access to specific equipment and may involve calibration and maintenance to ensure accuracy.
What is the significance of partial pressure in gas mixtures?
+The partial pressure of a gas in a mixture is significant because it determines the gas's contribution to the total pressure and its potential to react or interact with other components in the mixture. Understanding partial pressures is crucial in various chemical and physical processes.
How does temperature affect the partial pressure of a gas?
+Temperature affects the partial pressure of a gas according to the ideal gas law (PV = nRT). An increase in temperature, while keeping the volume and number of moles constant, will increase the pressure of the gas, including its partial pressure in a mixture.
Can partial pressure be negative?
+No, partial pressure cannot be negative. Pressure, by definition, is a measure of force per unit area and is always a positive quantity in the context of gas mixtures and the ideal gas law.
In conclusion, calculating the partial pressure of a gas in a mixture is fundamental in understanding and predicting the behavior of gases in various conditions. The methods outlined above, ranging from the application of Dalton’s law and the ideal gas law to the use of Henry’s law for dissolved gases, provide a comprehensive toolkit for determining partial pressures in different scenarios. Each method has its application and relevance, depending on the specific conditions and the information available. By mastering these techniques, one can better understand and work with gas mixtures in both theoretical and practical contexts.