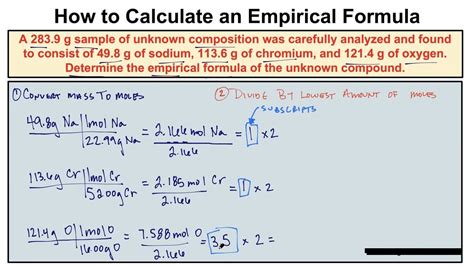
Obtaining the empirical formula of a compound can be a straightforward process if you have the right information and follow a step-by-step approach. The empirical formula is the simplest whole-number ratio of atoms of each element present in a compound. To get the empirical formula, you typically need to know the percentage composition of the compound, which can be determined through various analytical techniques such as combustion analysis.
Understanding the Concept of Empirical Formula

The empirical formula is a fundamental concept in chemistry that represents the simplest ratio of atoms of each element in a molecule. It is a crucial step in determining the molecular formula of a compound, which provides the actual number of atoms of each element in a molecule. Knowing the empirical formula helps in understanding the composition of a compound and is essential for further chemical analysis and synthesis.
Steps to Determine the Empirical Formula
Determining the empirical formula involves several steps, starting from the percentage composition of the elements in the compound. Here’s a simplified approach:
- Convert Percentage Composition to Grams: Assume you have 100 grams of the compound to simplify calculations. This means each element’s percentage composition directly translates to its mass in grams.
- Convert Grams to Moles: For each element, divide its mass in grams by its atomic mass to find the number of moles. This step is crucial because it allows us to work with the ratios of atoms rather than masses.
- Find the Simplest Ratio: Divide each of the mole values by the smallest number of moles to simplify the ratio. This will give you the empirical formula. If the ratios are not whole numbers, multiply all ratios by the smallest factor that will convert all to whole numbers.
| Element | Percentage Composition | Mass (g) for 100g Compound | Atomic Mass (g/mol) | Moles |
|---|---|---|---|---|
| Carbon (C) | 40% | 40g | 12.01 g/mol | 40 / 12.01 = 3.33 mol |
| Hydrogen (H) | 60% | 60g | 1.008 g/mol | 60 / 1.008 = 59.52 mol |

In this example, to find the simplest ratio, we divide each of the mole values by the smallest number of moles, which is 3.33 for carbon.
For Carbon: 3.33 mol / 3.33 = 1
For Hydrogen: 59.52 mol / 3.33 = 17.88, which simplifies to approximately 18 when considering whole numbers for empirical formulas.
This gives us an empirical formula of CH18, but to ensure this is correct, we must verify if the calculated empirical formula matches any known compounds or if further simplification is needed based on additional chemical information.
Practical Applications and Considerations
Understanding and determining the empirical formula has practical applications in various fields of chemistry, including organic chemistry, biochemistry, and materials science. It helps in identifying unknown compounds, synthesizing new materials, and understanding chemical reactions and processes.
Challenges and Limitations
While determining the empirical formula can be straightforward with precise percentage composition data, challenges arise when the data is not accurate or when dealing with compounds that have very similar atomic masses, making the calculation of moles and the subsequent ratio more complex.
Key Points
- The empirical formula is the simplest whole-number ratio of atoms of each element in a compound.
- Determining the empirical formula involves converting percentage composition to grams, then to moles, and finding the simplest ratio.
- The empirical formula is crucial for understanding the composition of a compound but may not directly provide the molecular formula.
- Accurate percentage composition data is essential for calculating the empirical formula.
- The empirical formula has significant practical applications in chemistry and materials science.
In conclusion, getting the empirical formula easily requires careful calculation and an understanding of the chemical principles involved. It is a fundamental concept in chemistry that offers valuable insights into the composition and properties of compounds, contributing to advancements in various scientific and technological fields.
What is the empirical formula, and why is it important?
+The empirical formula represents the simplest whole-number ratio of atoms of each element in a compound. It is important because it helps in understanding the composition of a compound, which is crucial for chemical analysis, synthesis, and understanding chemical reactions.
How do you calculate the empirical formula from percentage composition?
+To calculate the empirical formula, first convert the percentage composition to grams (assuming 100 grams of the compound), then convert grams to moles by dividing by the atomic mass of each element. Finally, find the simplest whole-number ratio of moles of each element.
What is the difference between the empirical and molecular formula?
+The empirical formula is the simplest whole-number ratio of atoms of each element in a compound, while the molecular formula shows the actual number of atoms of each element in a molecule. The molecular formula is a multiple of the empirical formula.