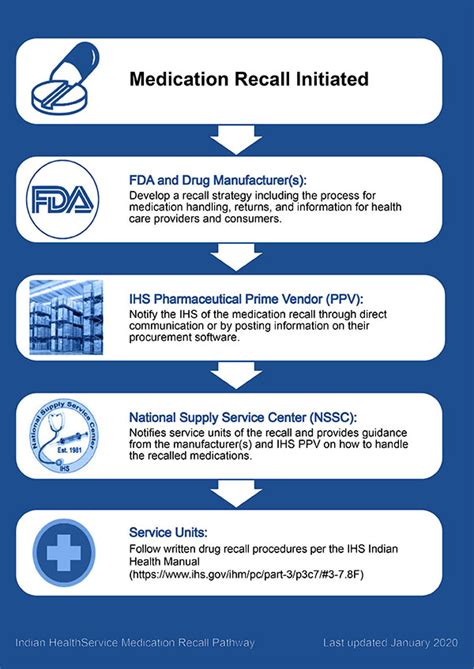
Medication recalls can be a daunting experience for patients, healthcare providers, and pharmaceutical companies alike. The complexity of managing recalls effectively is underscored by the need to balance patient safety with the logistical challenges of notifying and instructing affected parties. According to the U.S. Food and Drug Administration (FDA), medication recalls are actions taken by a firm to remove a product from the market due to a reason related to the product's safety or effectiveness. With the increasing number of medications on the market and the potential for errors or contamination, understanding how to handle medication recalls is more critical than ever.
Key Points
- Identifying the reasons behind medication recalls is crucial for preventing future occurrences.
- Prompt and clear communication with patients and healthcare providers is essential for effective recall management.
- Establishing a recall plan and conducting regular audits can help pharmaceutical companies and healthcare facilities prepare for and respond to recalls efficiently.
- Utilizing technology, such as automated notification systems, can enhance the recall process by ensuring timely and accurate information dissemination.
- Patient education and support are vital for minimizing the impact of medication recalls on public health.
Understanding Medication Recalls
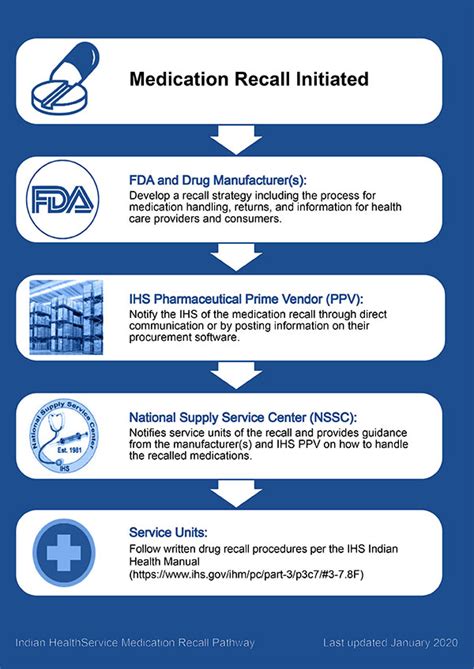
Medication recalls can be initiated by the FDA or voluntarily by the manufacturer due to various reasons, including contamination, manufacturing defects, or labeling errors. The classification of recalls by the FDA into three categories (I, II, and III) based on the level of risk to patients helps in prioritizing the response efforts. Class I recalls are the most serious and involve situations where there is a reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.
Preparation and Planning
Preparing for potential recalls is a proactive step that pharmaceutical companies and healthcare facilities can take to minimize disruptions and ensure patient safety. This includes developing a comprehensive recall plan that outlines procedures for notification, product retrieval, and patient support. Regular audits and inspections can help identify potential issues before they escalate into recalls. Furthermore, investing in quality control measures and employee training can reduce the likelihood of errors that could lead to recalls.
| Recall Classification | Description | Risk Level |
|---|---|---|
| Class I | Reasonable probability of serious adverse health consequences or death | High |
| Class II | May cause temporary or medically reversible adverse health consequences | Moderate |
| Class III | Not likely to cause adverse health consequences | Low |

Management and Communication
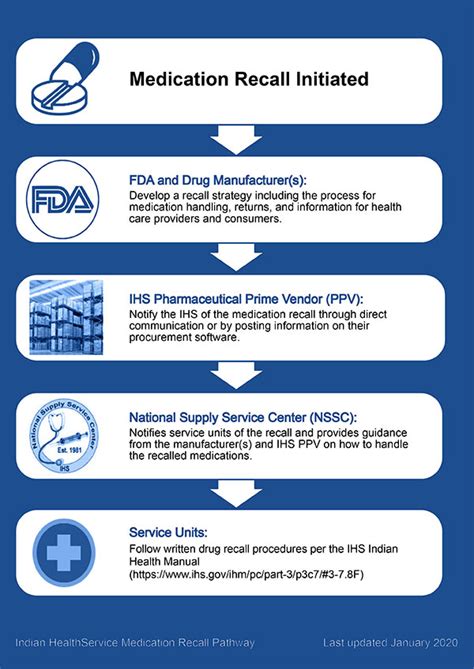
The management of medication recalls requires meticulous attention to detail and a systematic approach. Upon initiating a recall, the next steps involve notification of all relevant parties, including distributors, retailers, healthcare providers, and patients. The notification should be clear, concise, and timely, providing specific instructions on what actions to take. Utilizing multiple communication channels, such as mail, email, and public announcements, can help ensure that the information reaches all affected parties.
Supporting Patients
Patient support is a critical component of handling medication recalls. This includes providing alternative treatments or refunds as necessary, as well as addressing any concerns or questions patients may have. Patient education on the recall, its reasons, and the steps they need to take is essential for minimizing anxiety and ensuring compliance with recall instructions. Furthermore, offering ongoing support and monitoring for any potential health impacts can help mitigate the effects of the recall on patients.
In conclusion, handling medication recalls effectively is a multifaceted challenge that requires preparation, clear communication, and a patient-centric approach. By understanding the reasons behind recalls, developing comprehensive recall plans, and prioritizing patient support, we can work towards minimizing the impact of medication recalls on public health.
What are the primary reasons for medication recalls?
+Medication recalls can be due to contamination, manufacturing defects, labeling errors, or other safety and efficacy concerns.
How are medication recalls classified?
+The FDA classifies recalls into three categories (I, II, and III) based on the level of risk to patients, with Class I being the most serious.
What steps can patients take if their medication is recalled?
+Patient should follow the instructions provided with the recall notice, which may include stopping the medication, returning it to the pharmacy, or contacting their healthcare provider for alternative treatment options.