Understanding the charge of an element is fundamental in chemistry, as it determines how atoms interact with each other. The charge of an element, also known as its oxidation state, can be determined by following a set of straightforward rules. To begin, it's essential to recall that elements in their natural state have a charge of zero. This means that the number of protons (positive charges) in the nucleus is balanced by the number of electrons (negative charges) orbiting the nucleus.
Primary Rules for Determining Element Charge

The charge of an element can change when it forms compounds with other elements. In such cases, the charge is determined by the number of electrons gained or lost. Here are the primary rules to keep in mind:
- Metals tend to lose electrons, resulting in a positive charge. The charge of a metal ion is determined by the number of electrons it loses, which usually corresponds to the number of electrons in its outermost energy level.
- Nonmetals tend to gain electrons, resulting in a negative charge. The charge of a nonmetal ion is determined by the number of electrons it gains to fill its outermost energy level, typically to achieve a noble gas configuration.
Determining Charge Based on Electron Configuration
To determine the charge of an element based on its electron configuration, you need to know the number of electrons in the outermost energy level. For metals, the charge is usually equal to the number of electrons in the outermost energy level. For nonmetals, the charge is typically equal to the number of electrons needed to fill the outermost energy level to achieve a noble gas configuration.
| Element Type | Charge Determination |
|---|---|
| Metals | Number of electrons lost (usually equals the number of electrons in the outermost energy level) |
| Nonmetals | Number of electrons gained to fill the outermost energy level (usually to achieve a noble gas configuration) |

Common Charges of Elements
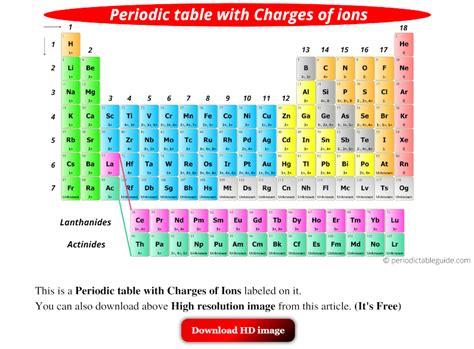
Certain elements exhibit common charges due to their electronic configurations. For example, alkali metals (Group 1) typically have a +1 charge, while alkaline earth metals (Group 2) have a +2 charge. Halogens (Group 17) usually have a -1 charge, and noble gases are unreactive and have a charge of 0.
Applying Valence Shell Electron Pair Repulsion (VSEPR) Theory
The VSEPR theory can help in understanding the shape of molecules and, by extension, the charge of elements within those molecules. By considering the number of electron pairs and lone pairs around a central atom, you can predict the molecular geometry and infer the charges of the atoms involved.
Key Points
- The charge of an element is determined by the number of electrons it gains or loses when forming compounds.
- Metals typically lose electrons to form positive ions, while nonmetals gain electrons to form negative ions.
- The electron configuration of an element can predict its charge, with metals losing electrons from their outermost energy level and nonmetals gaining electrons to fill their outermost energy level.
- Common charges can be predicted based on an element's position in the periodic table, such as +1 for alkali metals and -1 for halogens.
- The VSEPR theory can help understand molecular geometry and infer atomic charges within molecules.
In conclusion, determining the charge of an element involves understanding its electron configuration, position in the periodic table, and how it interacts with other elements to form compounds. By applying the rules outlined above and considering periodic trends, you can easily determine the charge of most elements.
How do metals and nonmetals differ in terms of charge?
+Metals tend to lose electrons, resulting in a positive charge, whereas nonmetals tend to gain electrons, resulting in a negative charge.
What role does electron configuration play in determining an element’s charge?
+Electron configuration helps predict the charge of an element by indicating the number of electrons in the outermost energy level. Metals lose electrons from this level, while nonmetals gain electrons to fill it.
Can the periodic table be used to predict common charges of elements?
+Yes, the periodic table can be used to predict common charges based on an element’s position. For example, alkali metals (Group 1) have a +1 charge, and halogens (Group 17) have a -1 charge.



