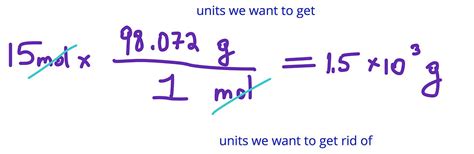Understanding the relationship between grams and moles is a fundamental concept in chemistry, crucial for calculating the quantities of substances involved in chemical reactions. The conversion between grams and moles involves the molar mass of a substance, which is the mass of one mole of that substance. This article aims to guide you through the process of converting grams to moles with ease, using step-by-step explanations and examples to solidify your understanding.
Key Points
- The molar mass of a substance is essential for converting between grams and moles.
- The formula to convert grams to moles is: moles = mass in grams / molar mass.
- Understanding the periodic table and how to calculate molar mass is crucial for accurate conversions.
- Practicing with examples helps in mastering the conversion process.
- Applications of grams to moles conversion are widespread in chemistry, including stoichiometry and chemical reactions.
Understanding Molar Mass
Molar mass is a critical concept in chemistry that represents the mass of one mole of a particular substance. It is expressed in units of grams per mole (g/mol). The molar mass of an element or compound can be found by summing the atomic masses of its constituent atoms, which are listed on the periodic table. For elements, the atomic mass is the average mass of the naturally occurring isotopes of that element. For compounds, the molar mass is calculated by adding the atomic masses of all the atoms in the compound’s formula.
Calculating Molar Mass of Compounds
Calculating the molar mass of a compound involves knowing its chemical formula and the atomic masses of its constituent elements. For example, to find the molar mass of sodium chloride (NaCl), you would add the atomic mass of sodium (Na) to the atomic mass of chlorine (Cl). If the atomic mass of sodium is approximately 22.99 g/mol and that of chlorine is approximately 35.45 g/mol, the molar mass of NaCl would be 22.99 g/mol + 35.45 g/mol = 58.44 g/mol.
| Element | Atomic Mass (g/mol) |
|---|---|
| Sodium (Na) | 22.99 |
| Chlorine (Cl) | 35.45 |
| Molar Mass of NaCl | 58.44 |
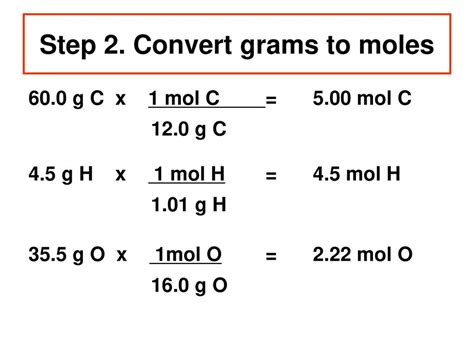
Converting Grams to Moles
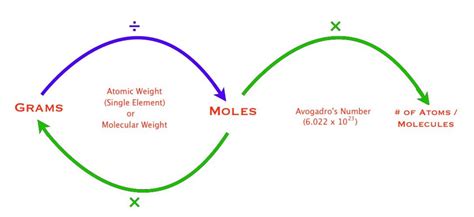
Once you have the molar mass of a substance, converting grams to moles is straightforward. The formula to use is: moles = mass in grams / molar mass. For instance, if you have 100 grams of sodium chloride (NaCl) and you know its molar mass is 58.44 g/mol, you can calculate the number of moles as follows: moles = 100 g / 58.44 g/mol.
Example Calculation
Let’s calculate the number of moles in 100 grams of NaCl using the molar mass calculated earlier (58.44 g/mol). Plugging the values into the formula gives: moles = 100 g / 58.44 g/mol = 1.71 mol. This means that 100 grams of sodium chloride is equivalent to 1.71 moles.
The conversion process is not only essential for understanding chemical reactions but also for applications in stoichiometry, where the quantitative relationship between reactants and products in chemical reactions is studied. Mastering the grams to moles conversion enables chemists to predict how much of each reactant is needed and how much product will be formed, based on the stoichiometric coefficients of the balanced chemical equation.
What is the importance of molar mass in chemistry?
+Molar mass is crucial for converting between grams and moles, allowing chemists to calculate the quantities of substances involved in chemical reactions accurately.
How do you calculate the molar mass of a compound?
+The molar mass of a compound is calculated by summing the atomic masses of all the atoms in the compound's formula, which can be found on the periodic table.
What is the formula for converting grams to moles?
+The formula to convert grams to moles is: moles = mass in grams / molar mass.
In conclusion, the conversion from grams to moles is a fundamental process in chemistry that relies on the molar mass of substances. By understanding how to calculate molar mass and apply it to convert between units, chemists can better analyze and predict the outcomes of chemical reactions. This knowledge is not just theoretical but has practical applications in fields such as stoichiometry, chemical synthesis, and materials science. As you continue to explore the world of chemistry, mastering the grams to moles conversion will become an indispensable tool in your toolkit.