The iron ion with 26 protons and 24 electrons is a fascinating example of atomic structure and chemical behavior. To understand this ion, we must first consider the basic composition of an iron atom. Iron, with the atomic number 26, naturally has 26 protons in its nucleus, which defines its position in the periodic table. However, the number of electrons in a neutral iron atom is also 26, distributed across various energy levels or shells around the nucleus.
Formation of the Iron Ion with 24 Electrons
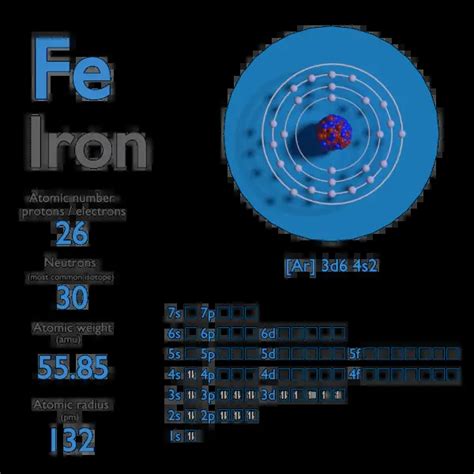
The ion in question has lost 2 electrons from its neutral state, resulting in a +2 charge. This process is known as ionization or ion formation. The loss of electrons typically occurs when an iron atom reacts with another substance that can accept electrons, such as oxygen during the formation of iron(II) oxide (FeO), or when it is in a solution where it can donate electrons, like in the case of iron salts. The resulting ion, Fe²⁺, has 26 protons (which remain unchanged) and 24 electrons, giving it a net positive charge of +2.
Electron Configuration and Stability
The electron configuration of the iron ion with 24 electrons can be derived from the electron configuration of the neutral iron atom, which is [Ar] 3d⁶ 4s². When iron loses 2 electrons to form the +2 ion, these electrons are typically lost from the 4s orbital, resulting in an electron configuration of [Ar] 3d⁶ for Fe²⁺. This configuration reflects the ion’s tendency to achieve a more stable state by filling its orbitals in a way that minimizes energy. The d⁶ configuration is particularly stable due to the half-filled d subshell, which contributes to the ion’s chemical properties and reactivity.
| Atomic Number | Number of Protons | Number of Electrons (Neutral) | Number of Electrons (Ion) |
|---|---|---|---|
| 26 | 26 | 26 | 24 |
Chemical Properties and Applications
The Fe²⁺ ion exhibits a range of chemical properties that make it useful in different applications. It can form compounds with various anions, such as chloride (FeCl₂), sulfate (FeSO₄), and carbonate (FeCO₃), which are used in industries like construction, water treatment, and pharmaceuticals. Additionally, the iron(II) ion is a key component in certain biochemical pathways, including the synthesis of hemoglobin and myoglobin, which are essential for oxygen transport and storage in living organisms.
Environmental and Health Implications
The presence of iron ions, including Fe²⁺, in the environment can have significant implications for both ecosystems and human health. Iron is an essential nutrient for many organisms, but excessive levels can lead to toxicity. In human health, iron deficiency is a common nutritional disorder, while iron overload can lead to conditions such as hemochromatosis. Understanding the role and behavior of iron ions in biological and environmental systems is crucial for managing these risks and benefits.
Key Points
- The iron ion with 26 protons and 24 electrons has a +2 charge, resulting from the loss of 2 electrons from the neutral iron atom.
- This ion has an electron configuration of [Ar] 3d⁶, which contributes to its stability and chemical properties.
- Fe²⁺ plays a critical role in biological systems, including oxygen transport and electron transfer processes.
- The ion forms various compounds used in industries like construction, water treatment, and pharmaceuticals.
- Understanding the properties and behaviors of the Fe²⁺ ion is essential for managing its environmental and health implications.
In conclusion, the iron ion with 26 protons and 24 electrons is a fundamental component of various chemical and biological processes. Its unique electron configuration and resulting chemical properties make it an essential element in both industrial applications and biological systems. As research continues to uncover the intricacies of iron chemistry and biology, our understanding of this ion's role in the natural world and its potential applications will only deepen.
What is the electron configuration of the iron(II) ion?
+The electron configuration of the iron(II) ion, Fe²⁺, is [Ar] 3d⁶, resulting from the loss of 2 electrons from the 4s orbital of the neutral iron atom.
What are some common applications of compounds containing the Fe²⁺ ion?
+Compounds containing the Fe²⁺ ion, such as iron(II) chloride, sulfate, and carbonate, are used in various industries, including construction, water treatment, and pharmaceuticals, due to their unique chemical properties.
Why is the iron(II) ion important in biological systems?
+The iron(II) ion plays a critical role in biological systems, particularly in the transport and storage of oxygen by hemoglobin and myoglobin, and in electron transfer processes during photosynthesis.