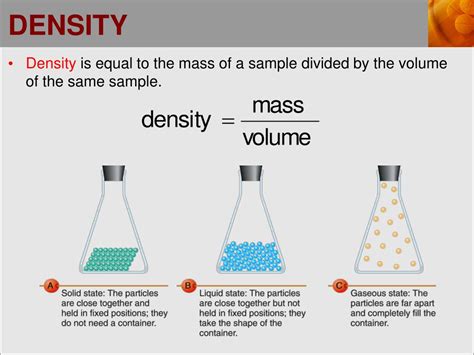
Density, a fundamental concept in physics, is defined as the amount of mass per unit volume of a substance. Understanding density is crucial in various fields, including engineering, chemistry, and materials science. The density of a material can significantly impact its behavior, properties, and applications. For instance, the density of water is approximately 1 gram per cubic centimeter (g/cm³), which is a reference point for comparing the densities of other substances. Materials with densities greater than that of water will sink, while those with lower densities will float.
Key Density Concepts

The concept of density is closely related to the atomic mass and the arrangement of atoms within a material. The density of a substance can be calculated using the formula: density = mass/volume. This calculation is straightforward for solid objects but can be more complex for fluids and gases due to their ability to change volume under different conditions. For example, the density of air varies with temperature and pressure, affecting its buoyancy and the efficiency of aircraft and wind turbines.
Density and Buoyancy
The principle of buoyancy, discovered by Archimedes, states that an object submerged in a fluid is buoyed up by a force equal to the weight of the fluid displaced by the object. This principle is directly related to density, as objects less dense than the surrounding fluid will float, while denser objects will sink. This concept is not only essential in understanding natural phenomena like the flotation of icebergs but also in designing ships, submarines, and other marine vessels. The density of seawater, approximately 1.03 g/cm³, is slightly higher than that of freshwater, which influences the buoyancy of objects in these environments.
| Substance | Density (g/cm³) |
|---|---|
| Air (at sea level) | 0.0012 |
| Water | 1.0 |
| Seawater | 1.03 |
| Iron | 7.9 |
| Lead | 11.34 |

Key Points
- Density is defined as mass per unit volume and is a critical property of substances that influences their buoyancy and behavior under different conditions.
- The density of water serves as a reference point for comparing the densities of other substances, with materials denser than water sinking and those less dense floating.
- Calculating density involves dividing the mass of a substance by its volume, a process that can be complex for fluids and gases due to their variable volumes.
- Understanding density is essential for designing and operating vehicles like ships and submarines, as well as for predicting natural phenomena such as the behavior of icebergs.
- Manipulating density can lead to advancements in materials science and engineering, enabling the creation of materials with unique properties tailored to specific applications.
Density in Nature and Industry

Density plays a significant role in natural phenomena and industrial applications. In geology, the density of minerals and rocks is crucial for understanding the Earth’s structure and processes. The Earth’s core, with a density of about 13 g/cm³, is significantly denser than the crust, which has a density ranging from 2.5 to 3.0 g/cm³. This difference in density affects the Earth’s gravitational field and the movement of tectonic plates. In industry, controlling density is vital in the production of metals, where small variations can significantly impact the material’s strength, conductivity, and durability.
Applications of Density Manipulation
Manipulating density can lead to the creation of materials with unique properties. For example, foams and porous materials have lower densities than their solid counterparts, which can be beneficial in applications requiring lightness and insulation. Aerogels, with densities as low as 0.1 g/cm³, are used in thermal insulation and have potential applications in aerospace engineering. Conversely, high-density materials are crucial in applications requiring strength and durability, such as in the construction of high-rise buildings and bridges.
In conclusion, density is a fundamental property that underpins many aspects of science and engineering. From the buoyancy of objects in fluids to the design of advanced materials, understanding and manipulating density are essential skills for professionals across various disciplines. As research continues to uncover new ways to control and utilize density, we can expect significant advancements in technology and our understanding of the natural world.
What is the relationship between density and buoyancy?
+The principle of buoyancy states that an object will float if it is less dense than the surrounding fluid and sink if it is denser. This relationship is fundamental in understanding why certain objects float or sink in water or air.
How is density calculated?
+Density is calculated by dividing the mass of an object by its volume. For solids, this calculation is straightforward, but for fluids and gases, it can be more complex due to their variable volumes under different conditions.
What are some practical applications of understanding density?
+Understanding density has numerous practical applications, including the design of ships and submarines, the prediction of natural phenomena like the behavior of icebergs, and the development of new materials with unique properties for various industries.