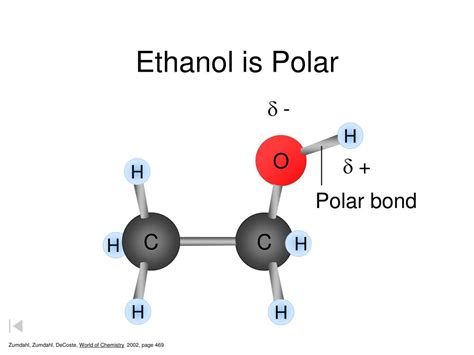
The question of whether ethanol is polar or not is a fundamental concept in chemistry, particularly in understanding the properties and behaviors of molecules. Ethanol, with the chemical formula C2H5OH, is a compound that consists of a hydroxyl group (-OH) attached to a carbon chain. The presence of this hydroxyl group is crucial in determining the polarity of ethanol.
Understanding Polarity
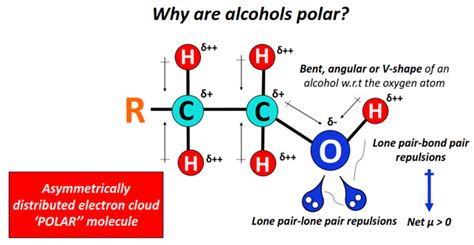
Polarity in chemistry refers to the separation of electric charge within a molecule, resulting in a molecule or its chemical groups having an electric dipole moment. This separation of charge occurs due to the difference in electronegativity between atoms in a covalent bond. Electronegativity is a measure of an atom’s ability to attract and hold onto electrons in a bond.
Electronegativity and Ethanol
In the case of ethanol, the oxygen atom in the hydroxyl group is more electronegative than the hydrogen atoms and the carbon atoms. This means that the oxygen atom tends to pull the shared electrons closer to itself, creating a partial negative charge (δ-) on the oxygen atom and a partial positive charge (δ+) on the hydrogen atom of the hydroxyl group. This separation of charge within the molecule is what gives ethanol its polar characteristic.
| Atom | Electronegativity Value |
|---|---|
| Oxygen (O) | 3.44 |
| Hydrogen (H) | 2.20 |
| Carbon (C) | 2.55 |
As shown in the table, oxygen has a significantly higher electronegativity value compared to hydrogen and carbon, which supports the reasoning behind the polarity of ethanol.
Implications of Polarity
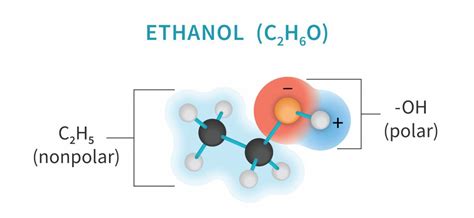
The polarity of ethanol has several implications for its behavior and interactions. For example, ethanol is miscible with water due to its ability to form hydrogen bonds with water molecules. This property is crucial in many industrial and biological processes. Additionally, the polarity of ethanol influences its boiling point; it has a relatively high boiling point compared to non-polar molecules of similar molecular weight, due to the stronger intermolecular forces (hydrogen bonding) between ethanol molecules.
Practical Applications
In practical terms, the polarity of ethanol is exploited in various applications. For instance, in the pharmaceutical industry, ethanol is used as a solvent for many medications due to its polar nature, which allows it to dissolve a wide range of substances. Similarly, in biochemistry, ethanol’s polarity is crucial for its role in metabolic pathways and as a product of fermentation.
Key Points
- Ethanol is considered a polar molecule due to the presence of the hydroxyl (-OH) group, which creates a separation of electric charge within the molecule.
- The oxygen atom in the hydroxyl group is more electronegative than the hydrogen and carbon atoms, leading to a partial negative charge on the oxygen and a partial positive charge on the hydrogen of the hydroxyl group.
- The polarity of ethanol affects its physical and chemical properties, such as its boiling point, solubility, and ability to form hydrogen bonds.
- Ethanol's polarity is crucial for its applications as a solvent, a fuel, and in the production of alcoholic beverages.
- The ability of ethanol to form hydrogen bonds with water molecules makes it miscible with water, which is important for various industrial and biological processes.
In conclusion, the polarity of ethanol is a fundamental aspect of its chemistry, influencing its properties, behaviors, and applications. Understanding the polar nature of ethanol is essential for appreciating its role in various chemical, biological, and industrial contexts.
What makes ethanol polar?
+Ethanol is polar due to the hydroxyl (-OH) group, where the oxygen atom is more electronegative than the hydrogen and carbon atoms, creating a separation of electric charge within the molecule.
How does the polarity of ethanol affect its properties?
+The polarity of ethanol influences its boiling point, solubility in water, and its ability to form hydrogen bonds, making it useful in various applications such as a solvent, a fuel, and in the production of alcoholic beverages.
Why is ethanol miscible with water?
+Ethanol is miscible with water because of its ability to form hydrogen bonds with water molecules, which is a result of its polar nature.