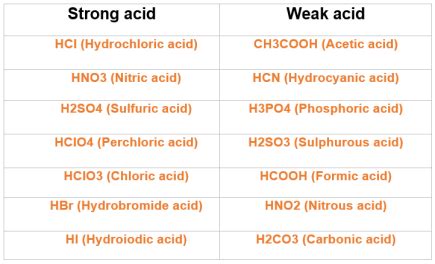
The classification of an acid as strong or weak is based on its ability to completely dissociate in water, producing hydrogen ions (H+) and the conjugate base of the acid. Nitric acid, denoted by the chemical formula HNO3, is a common acid used in various industrial and laboratory applications. Its strength as an acid is a fundamental aspect of its chemical properties.
Definition of a Strong Acid

A strong acid is defined as an acid that completely dissociates or ionizes in aqueous solution, meaning it fully breaks down into its constituent ions. This complete dissociation results in a high concentration of hydrogen ions (H+) in the solution, which is a characteristic feature of strong acids. The dissociation reaction of a strong acid can be represented by the general equation: HA → H+ + A-, where HA is the acid and A- is its conjugate base.
Dissociation of HNO3
Nitric acid (HNO3) is known to dissociate in water according to the following equation: HNO3 → H+ + NO3-. This dissociation is essentially complete, indicating that nitric acid is a strong acid. The nitrate ion (NO3-) is the conjugate base of nitric acid. The complete dissociation of HNO3 in water is supported by its high dissociation constant (Ka), which is a quantitative measure of the strength of an acid in solution.
| Acid | Ka Value |
|---|---|
| HNO3 (Nitric Acid) | Very large, indicating complete dissociation |
| HCl (Hydrochloric Acid) | 1.3 × 10^6 |
| H2SO4 (Sulfuric Acid) | Very large for the first dissociation step |
Chemical Properties and Applications
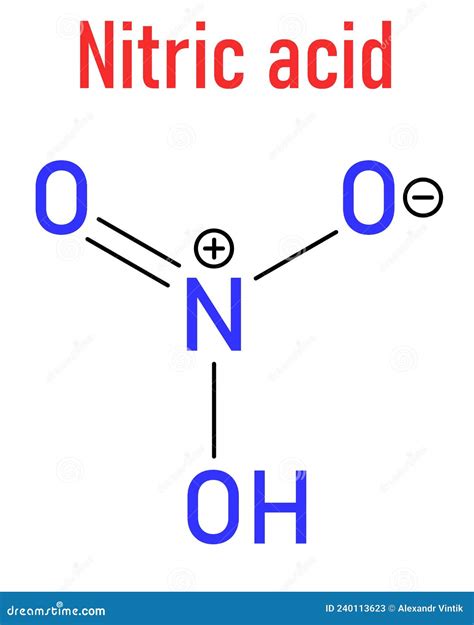
The strong acid nature of HNO3 influences its chemical properties and applications. It is highly corrosive and can dissolve many metals, releasing hydrogen gas and forming nitrate salts. This property makes it useful for metal etching and cleaning. Additionally, its ability to completely dissociate in water contributes to its effectiveness as an oxidizing agent, which is essential for its role in the production of nitrate salts and other compounds.
Environmental and Safety Considerations
Given its strong acidic nature, handling HNO3 requires careful attention to safety to avoid skin and eye damage, as well as inhalation of its fumes. Environmental considerations are also important, as improper disposal of nitric acid can lead to water pollution and harm aquatic life.
Key Points
- Nitric acid (HNO3) is classified as a strong acid due to its complete dissociation in water.
- Its dissociation reaction is HNO3 → H+ + NO3-, producing a high concentration of hydrogen ions.
- The strength of HNO3 is indicated by its high dissociation constant (Ka) value.
- Its applications include metal etching, production of fertilizers and explosives, and as a laboratory reagent.
- Handling and disposal of HNO3 require strict safety and environmental precautions.
In conclusion, the strong acid nature of HNO3 is a critical aspect of its chemical properties and applications. Understanding its strength and how it behaves in aqueous solutions is essential for its safe and effective use across various industries.
What makes HNO3 a strong acid?
+HNO3 is considered a strong acid because it completely dissociates in water, producing a high concentration of hydrogen ions (H+) and its conjugate base, the nitrate ion (NO3-).
What are the common applications of nitric acid?
+Nitric acid is used in the production of fertilizers, explosives, and as a laboratory reagent. It is also used for metal etching and cleaning due to its corrosive properties.
Why is safety important when handling HNO3?
+Safety is crucial when handling HNO3 because it is highly corrosive and can cause severe burns upon contact with skin. Inhaling its fumes can also be harmful. Additionally, improper disposal can lead to environmental pollution.