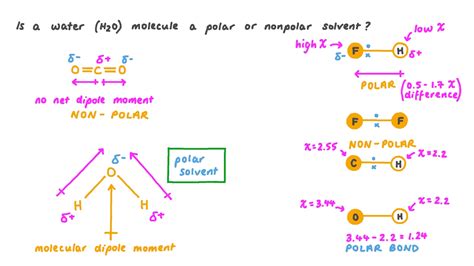
Water, with its chemical formula H2O, is a molecule that has been extensively studied due to its unique properties and essential role in various biological, chemical, and physical processes. One of the key characteristics of water that has sparked interest and debate is its polarity. Understanding whether water is nonpolar or polar requires a deep dive into its molecular structure and the principles of chemistry that define polarity.
Understanding Polarity in Molecules
Polarity in a molecule is determined by the distribution of electric charge within the molecule. A molecule is considered polar if it has a net dipole moment, meaning there is a separation of positive and negative charges. This separation occurs when there is a difference in electronegativity between the atoms in a covalent bond, leading to an unequal sharing of electrons. On the other hand, a molecule is nonpolar if it does not have a net dipole moment, indicating that the electrons are shared more equally between the atoms, or the molecule’s shape is symmetrical, canceling out any individual bond dipoles.
The Molecular Structure of Water
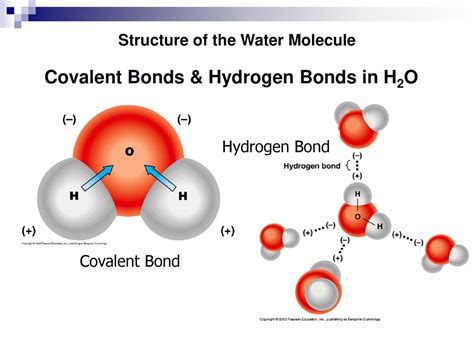
Water is composed of two hydrogen atoms and one oxygen atom. The oxygen atom is more electronegative than the hydrogen atoms, meaning it has a greater tendency to attract electrons towards itself. In the water molecule, the oxygen atom pulls the shared electrons closer to itself, resulting in a partial negative charge on the oxygen and partial positive charges on the hydrogen atoms. This creates a dipole moment, with the oxygen end of the molecule being slightly negative and the hydrogen ends being slightly positive.
| Atom | Electronegativity |
|---|---|
| Oxygen (O) | 3.44 |
| Hydrogen (H) | 2.20 |
This difference in electronegativity and the resulting partial charges give water its polar characteristic. The polarity of water is crucial for its role in biological systems, including its ability to dissolve a wide variety of substances, participate in chemical reactions, and regulate temperature and weather patterns.
Key Points
Key Points
- Water’s molecular structure, with two hydrogen atoms and one oxygen atom, is crucial for determining its polarity.
- The difference in electronegativity between oxygen and hydrogen atoms leads to a partial negative charge on the oxygen and partial positive charges on the hydrogens, creating a dipole moment.
- The polarity of water is essential for its solubility, participation in chemical reactions, and its role in biological and environmental processes.
- Understanding the polarity of water requires a grasp of chemical principles, including electronegativity and the distribution of electric charge within a molecule.
- Water’s unique properties, partly due to its polarity, make it vital for life as we know it.
Implications of Water’s Polarity
The polarity of water has significant implications for its physical and chemical properties. For instance, water’s high surface tension, which allows it to resist external forces and maintain its shape against gravity, is partly due to the hydrogen bonding between water molecules. This hydrogen bonding is a direct result of water’s polarity, where the slightly positive hydrogen atoms of one water molecule are attracted to the slightly negative oxygen atoms of another. Furthermore, water’s polarity enables it to dissolve a wide range of substances, from salts and minerals to gases like oxygen and carbon dioxide, which is crucial for aquatic life and various industrial applications.
In conclusion, water is indeed a polar molecule due to the unequal sharing of electrons between its oxygen and hydrogen atoms, leading to a net dipole moment. This polarity is fundamental to understanding water's unique properties and its central role in chemical, biological, and environmental processes.
What makes a molecule polar or nonpolar?
+A molecule is considered polar if there is a significant difference in electronegativity between the atoms in a covalent bond, leading to an unequal sharing of electrons and a net dipole moment. If the molecule’s shape is symmetrical or the electrons are shared more equally, it is considered nonpolar.
Why is the polarity of water important?
+The polarity of water is crucial for its ability to dissolve substances, participate in chemical reactions, and regulate temperature, among other roles. It underpins many of water’s unique properties that are essential for life and various industrial processes.
Can water’s polarity be altered?
+While the inherent polarity of a water molecule cannot be changed, the physical properties of water can be influenced by external factors such as temperature and pressure. However, these changes do not alter the fundamental polarity of the water molecule itself.