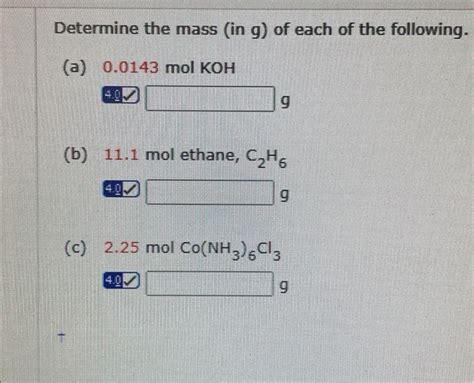
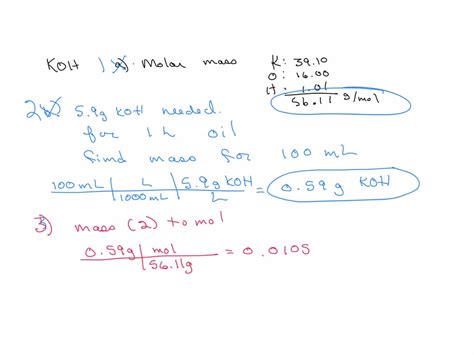Koh, or potassium hydroxide, is a strong base with a wide range of applications in various industries, including chemistry, manufacturing, and pharmaceuticals. Understanding the molar mass of Koh is crucial for calculating the quantities required for specific reactions or processes. The molar mass of a substance is the mass of one mole of that substance, expressed in grams per mole (g/mol). For Koh, the molar mass can be calculated by summing the atomic masses of its constituent elements: potassium (K), oxygen (O), and hydrogen (H).
Calculating the Molar Mass of Koh
The atomic masses of the elements in Koh are approximately: potassium (K) = 39.09 g/mol, oxygen (O) = 16.00 g/mol, and hydrogen (H) = 1.01 g/mol. To calculate the molar mass of Koh (KOHH), we sum these values: 39.09 (for K) + 16.00 (for O) + 1.01 (for H) = 56.10 g/mol. Therefore, the molar mass of Koh is approximately 56.10 g/mol.
Understanding Molar Mass in Chemical Reactions
In chemical reactions, the molar mass of reactants and products is critical for determining the stoichiometry of the reaction, which is the quantitative relationship between the reactants and products. Knowing the molar mass of Koh allows chemists to calculate how much of the substance is needed to react with another substance to produce a certain amount of product, based on the balanced chemical equation for the reaction.
| Element | Atomic Mass (g/mol) |
|---|---|
| Potassium (K) | 39.09 |
| Oxygen (O) | 16.00 |
| Hydrogen (H) | 1.01 |
| Koh (KOH) | 56.10 |
Applications of Koh in Industry
Koh has numerous applications in various industries. It is used in the manufacture of soap, glass, and paper. In the pharmaceutical industry, Koh is used as an intermediate in the production of certain drugs. Its strong basic properties make it useful for neutralizing acids and for the synthesis of other compounds.
Importance of Accurate Molar Mass Calculations
Accurate calculations of the molar mass of substances like Koh are essential for ensuring the quality and safety of products. Incorrect calculations can lead to improper mixing of chemicals, potentially resulting in dangerous reactions or the production of harmful by-products. Thus, understanding and correctly applying the concept of molar mass is a critical skill in chemistry and related fields.
Key Points
- The molar mass of Koh is calculated by summing the atomic masses of potassium, oxygen, and hydrogen, resulting in approximately 56.10 g/mol.
- Understanding the molar mass of substances is crucial for calculating stoichiometry in chemical reactions.
- Koh has various industrial applications, including the manufacture of soap, glass, paper, and pharmaceuticals.
- Accurate molar mass calculations are essential for product quality and safety.
- The concept of molar mass is fundamental in chemistry for predicting reaction outcomes and manufacturing processes.
In conclusion, the molar mass of Koh, approximately 56.10 g/mol, is a critical piece of information for chemists and industries that utilize this strong base. Its applications are diverse, ranging from manufacturing to pharmaceuticals, and accurate calculations of its molar mass are essential for safe and effective use.
What is the molar mass of Koh, and how is it calculated?
+The molar mass of Koh is approximately 56.10 g/mol, calculated by summing the atomic masses of potassium (39.09 g/mol), oxygen (16.00 g/mol), and hydrogen (1.01 g/mol).
Why is understanding the molar mass of substances like Koh important in chemistry?
+Understanding the molar mass of substances like Koh is important for calculating the quantities required for specific reactions, ensuring the correct stoichiometry, and predicting the outcomes of chemical reactions.
What are some industrial applications of Koh?
+Koh is used in the manufacture of soap, glass, paper, and in the pharmaceutical industry as an intermediate for the production of certain drugs.