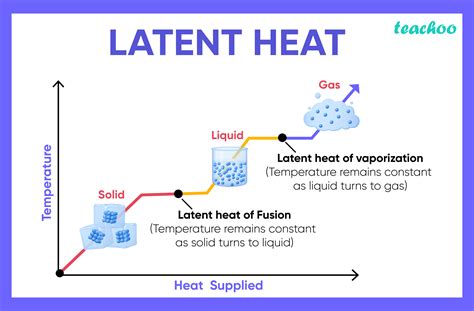
Latent heat, a fundamental concept in thermodynamics, refers to the amount of heat energy required to change the state of a substance without altering its temperature. This phenomenon is crucial in various natural and industrial processes, including weather patterns, refrigeration, and power generation. Understanding latent heat is essential for optimizing systems that involve phase changes, such as melting, freezing, vaporization, or condensation. In this article, we will delve into the world of latent heat, exploring its principles, applications, and providing valuable tips for those seeking to grasp or apply this concept in their studies or professional pursuits.
Key Points
- Latent heat plays a critical role in weather patterns and climate regulation.
- Understanding latent heat is essential for the design and optimization of refrigeration and air conditioning systems.
- Latent heat of vaporization and condensation are key factors in the efficiency of steam power plants.
- Materials with high latent heat capacity can be used for thermal energy storage.
- Accurate measurement and calculation of latent heat are crucial for predicting and managing phase change processes.
Understanding Latent Heat
Latent heat is categorized into two main types: latent heat of fusion (associated with solid-liquid phase changes) and latent heat of vaporization (associated with liquid-gas phase changes). The latent heat of a substance is a material property that can be measured experimentally or found in thermodynamic tables. For instance, the latent heat of fusion for ice is approximately 334 J/g, meaning that 334 joules of energy are required to melt one gram of ice at 0°C without changing its temperature.
Applications of Latent Heat
One of the most significant applications of latent heat is in refrigeration systems. Refrigerators and air conditioners work by exploiting the principle of latent heat of vaporization. A refrigerant, which has a low boiling point, absorbs heat from the interior of the refrigerator or the room and vaporizes, carrying the heat away. This vapor then condenses back into a liquid outside, releasing the heat to the surroundings. This process is highly efficient due to the high latent heat of vaporization of the refrigerant, allowing for effective cooling with minimal energy input.
| Substance | Latent Heat of Fusion (J/g) | Latent Heat of Vaporization (J/g) |
|---|---|---|
| Water | 334 | 2257 |
| Ethanol | 104 | 855 |
| Ammonia | 332 | 1370 |

Calculating Latent Heat

The calculation of latent heat involves understanding the amount of heat energy transferred during a phase change. The formula for latent heat (Q) is given by Q = mL, where m is the mass of the substance and L is the latent heat of the substance. For example, to calculate the energy required to melt 1 kg of ice, we use Q = 1 kg * 334 J/g = 334,000 J or 334 kJ, considering the latent heat of fusion for ice is 334 J/g.
Importance in Daily Life
Latent heat plays a vital role in our daily lives, from the functioning of refrigerators and air conditioners to the formation of rain and the Earth’s climate system. In meteorology, the latent heat released when water vapor condenses into clouds drives many weather phenomena, including storms and precipitation patterns. Moreover, the study of latent heat is crucial for predicting and mitigating the effects of climate change, as it influences the Earth’s energy balance and temperature regulation.
What is the difference between latent heat and sensible heat?
+Latent heat is the energy absorbed or released during a phase change without a change in temperature, whereas sensible heat is the energy required to change the temperature of a substance without a phase change.
How does latent heat affect the efficiency of power plants?
+The latent heat of vaporization and condensation of water is crucial in steam power plants. Water is heated to produce steam, which then expands through turbines to generate electricity. The efficiency of this process is significantly influenced by the latent heat of vaporization of water, as it determines how much energy can be extracted from the steam.
Can latent heat be used for energy storage?
+Yes, materials with high latent heat capacity can be used for thermal energy storage. Phase change materials (PCMs) absorb and release thermal energy during the melting and solidifying process, respectively, without significant changes in temperature. This property makes them suitable for applications in buildings, where they can help regulate indoor temperatures and reduce the need for air conditioning and heating.
In conclusion, latent heat is a fundamental principle that underlies many natural and industrial processes. Its applications range from the design of refrigeration systems and power plants to the understanding of weather patterns and climate change. By grasping the concept of latent heat and its implications, we can better appreciate the intricacies of our physical world and contribute to the development of more efficient and sustainable technologies.