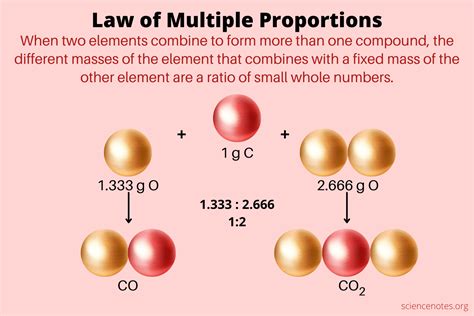
The Law of Multiple Proportions, also known as the Law of Definite Proportions or Dalton's Law, is a fundamental principle in chemistry that describes the relationship between the elements in a compound. This law states that when two elements combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element are in simple whole-number ratios. In other words, if two elements, A and B, form two different compounds, the ratio of the mass of A to the mass of B in the first compound is a simple whole-number ratio of the ratio of the mass of A to the mass of B in the second compound.
This concept was first proposed by John Dalton in the early 19th century and has since become a cornerstone of chemical theory. The Law of Multiple Proportions is often used in conjunction with other chemical principles, such as the Law of Conservation of Mass and the Law of Definite Proportions, to understand the composition and properties of chemical compounds. By applying this law, chemists can determine the empirical formulas of compounds and predict the properties of unknown substances.
Key Points
- The Law of Multiple Proportions states that the masses of one element that combine with a fixed mass of another element are in simple whole-number ratios.
- This law applies to compounds formed by two elements and is used to determine empirical formulas and predict properties of unknown substances.
- The Law of Multiple Proportions is a fundamental principle in chemistry, proposed by John Dalton in the early 19th century.
- Understanding this law is essential for chemists to analyze and synthesize compounds, as well as to predict their properties and behaviors.
- The Law of Multiple Proportions is often used in conjunction with other chemical principles, such as the Law of Conservation of Mass and the Law of Definite Proportions.
Historical Background and Development
The Law of Multiple Proportions was first proposed by John Dalton, an English chemist and physicist, in the early 19th century. At that time, Dalton was working on a theory of atomism, which posited that elements are composed of small, indivisible particles called atoms. Through his experiments and observations, Dalton discovered that when two elements combine to form different compounds, the masses of one element that combine with a fixed mass of the other element are in simple whole-number ratios.
Dalton's discovery of the Law of Multiple Proportions was a major breakthrough in the field of chemistry, as it provided a fundamental principle for understanding the composition and properties of chemical compounds. The law has since been widely accepted and is now considered a cornerstone of chemical theory. Over the years, the Law of Multiple Proportions has been refined and expanded upon by other scientists, who have used it to develop new theories and models of chemical bonding and reactivity.
Examples and Applications
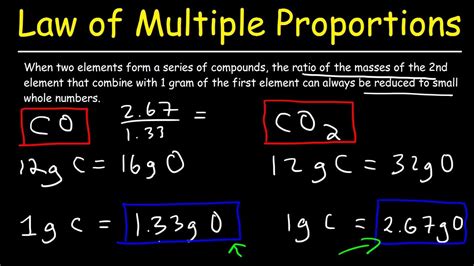
The Law of Multiple Proportions has numerous applications in chemistry, from determining the empirical formulas of compounds to predicting the properties of unknown substances. For example, consider the compounds carbon monoxide (CO) and carbon dioxide (CO2). In CO, one atom of carbon combines with one atom of oxygen, while in CO2, one atom of carbon combines with two atoms of oxygen. The ratio of the mass of oxygen to the mass of carbon in CO is 1:1, while the ratio in CO2 is 2:1, illustrating the simple whole-number ratio predicted by the Law of Multiple Proportions.
| Compound | Formula | Mass Ratio |
|---|---|---|
| Carbon Monoxide | CO | 1:1 |
| Carbon Dioxide | CO2 | 2:1 |

Implications and Limitations
While the Law of Multiple Proportions is a fundamental principle in chemistry, it is not without its limitations. The law assumes that the compounds in question are composed of simple, whole-number ratios of atoms, which is not always the case. Additionally, the law does not account for the presence of impurities or defects in the compounds, which can affect their properties and behaviors.
Despite these limitations, the Law of Multiple Proportions remains a cornerstone of chemical theory, providing a framework for understanding the composition and properties of chemical compounds. By applying this law, chemists can gain insights into the underlying structure and reactivity of substances, which is essential for developing new materials, designing new chemical processes, and predicting the properties of unknown substances.
Critical Evaluation and Future Directions
The Law of Multiple Proportions has undergone significant refinement and expansion since its initial proposal by Dalton. Modern chemistry has revealed the complexity of chemical bonding and the importance of factors such as electron configuration, molecular orbitals, and intermolecular forces. Despite these advances, the Law of Multiple Proportions remains a fundamental principle, providing a framework for understanding the composition and properties of chemical compounds.
Future research directions may involve the development of new theories and models that incorporate the Law of Multiple Proportions, as well as the exploration of its limitations and potential applications in fields such as materials science and nanotechnology. By continuing to refine and expand our understanding of this law, chemists can develop new insights into the underlying structure and reactivity of substances, leading to the discovery of new materials and the design of new chemical processes.
What is the Law of Multiple Proportions?
+The Law of Multiple Proportions states that when two elements combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element are in simple whole-number ratios.
What are the implications of the Law of Multiple Proportions?
+The Law of Multiple Proportions provides a framework for understanding the composition and properties of chemical compounds, allowing chemists to predict the properties and behaviors of unknown substances and develop new theories and models of chemical bonding.
What are the limitations of the Law of Multiple Proportions?
+The Law of Multiple Proportions assumes that the compounds in question are composed of simple, whole-number ratios of atoms, which is not always the case. Additionally, the law does not account for the presence of impurities or defects in the compounds, which can affect their properties and behaviors.