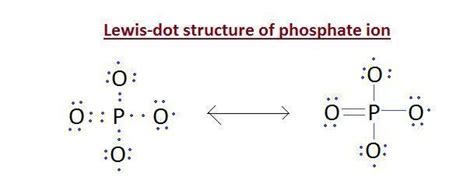
The Lewis structure of phosphate, which is a polyatomic ion with the chemical formula PO4^3-, is a crucial concept in chemistry that helps in understanding the arrangement of electrons and bonds within the molecule. To draw the Lewis structure of phosphate, one must follow a series of steps that involve determining the total number of valence electrons, drawing the skeleton structure, and then distributing the electrons to satisfy the octet rule for each atom. Here are five key ways to approach the Lewis structure of phosphate:
Key Points
- Determine the total number of valence electrons in the phosphate ion.
- Draw the skeleton structure of phosphate, with phosphorus as the central atom.
- Distribute the electrons to form single bonds between phosphorus and each oxygen atom.
- Use lone pairs on oxygen atoms to satisfy the octet rule for each atom.
- Consider the formal charges on each atom to ensure they are minimized and the structure is stable.
Understanding Phosphate’s Valence Electrons
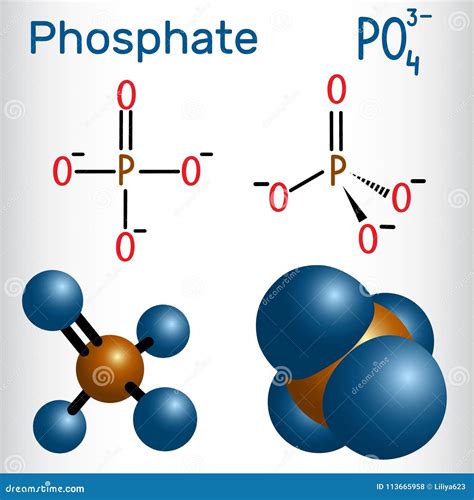
Phosphorus, being in group 15 of the periodic table, has 5 valence electrons. Oxygen, in group 16, has 6 valence electrons. Since there are four oxygen atoms in phosphate, the total number of valence electrons from oxygen is 4 * 6 = 24. Adding the 5 valence electrons from phosphorus gives a total of 29 valence electrons. However, phosphate has a -3 charge, meaning it has 3 additional electrons, bringing the total to 32 valence electrons.
Drawing the Skeleton Structure
The skeleton structure of phosphate is drawn with phosphorus (P) as the central atom, and the four oxygen atoms (O) surrounding it. This structure is based on the principle that the central atom is the one that can form the most bonds, which in this case is phosphorus due to its lower electronegativity compared to oxygen.
The Lewis structure starts with single bonds between phosphorus and each of the four oxygen atoms. This accounts for 8 electrons (2 electrons per bond). Each oxygen atom then receives 6 additional electrons to fill its octet, except for one or more oxygen atoms which might form a double bond with phosphorus to satisfy the octet rule for phosphorus and minimize formal charges.
Formal Charges and Stability
When drawing the Lewis structure, it’s crucial to consider the formal charges on each atom. The formal charge of an atom in a Lewis structure is calculated as the number of valence electrons in the free atom minus the number of non-bonding electrons and half the number of bonding electrons. A structure with lower formal charges, especially on the more electronegative atoms, is generally more stable.
In the case of phosphate, to minimize formal charges, one might draw a structure with one or more double bonds between phosphorus and oxygen. However, because phosphate is a resonance hybrid, the actual structure involves the delocalization of electrons over all four oxygen atoms, resulting in four equivalent bonds and no net formal charge on any atom when considering the resonance structures together.
Resonance Structures
The phosphate ion exhibits resonance, meaning its Lewis structure cannot be represented by a single structure. Instead, several structures are drawn, each representing a possible arrangement of electrons. These structures are then considered to contribute to a hybrid structure, which represents the actual molecule. For phosphate, this means drawing structures with a double bond between phosphorus and one of the oxygens, then moving this double bond to each of the other oxygens in subsequent structures, while also considering the formal charges and ensuring each atom has a full octet where possible.
| Atom | Valence Electrons | Contribution to Phosphate |
|---|---|---|
| Phosphorus (P) | 5 | Central atom, forms bonds with 4 oxygens |
| Oxygen (O) | 6 | Forms single or double bonds with P, satisfies octet with lone pairs |
| Charge on Phosphate | -3 | 3 additional electrons beyond neutral molecule |
Conclusion and Implications
In conclusion, the Lewis structure of phosphate is a foundational concept in chemistry that illustrates the arrangement of electrons and bonds within the phosphate ion. By understanding how to draw and interpret the Lewis structure, including the distribution of electrons, the formation of bonds, and the consideration of formal charges, chemists can better predict the behavior of phosphate in various chemical reactions and environments. This knowledge has implications for fields ranging from biochemistry, where phosphate groups play critical roles in the structure and function of biomolecules, to materials science, where phosphates are used in the development of new materials.
What is the total number of valence electrons in the phosphate ion?
+The phosphate ion (PO4^3-) has a total of 32 valence electrons: 5 from phosphorus, 24 from the four oxygen atoms, and 3 additional electrons due to the -3 charge.
Why is phosphorus the central atom in the phosphate ion?
+Phosphorus is the central atom because it can form the most bonds. In the phosphate ion, phosphorus is bonded to four oxygen atoms, which is possible due to its lower electronegativity compared to oxygen and its ability to expand its octet.
What is the significance of resonance in the phosphate ion’s Lewis structure?
+The resonance in the phosphate ion’s Lewis structure indicates that the actual structure is a hybrid of several resonance structures. This delocalization of electrons contributes to the stability of the phosphate ion and is crucial for understanding its chemical properties and reactivity.