Acids and bases are fundamental concepts in chemistry, and understanding their properties is crucial for various applications in fields such as biology, medicine, and environmental science. Among the numerous acids and bases, there are seven strong acids and bases that are widely recognized for their complete dissociation in water, resulting in a high concentration of hydrogen ions (H+) or hydroxide ions (OH-). In this article, we will delve into the properties, characteristics, and applications of these seven strong acids and bases, exploring their significance in various chemical reactions and processes.
Key Points
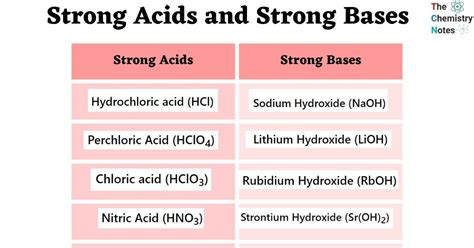
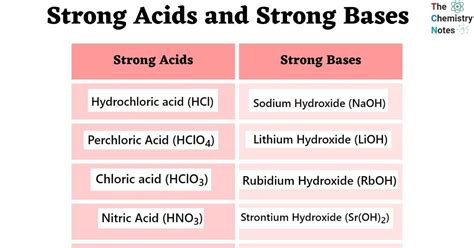
- The seven strong acids are hydrochloric acid (HCl), hydrobromic acid (HBr), hydroiodic acid (HI), sulfuric acid (H2SO4), nitric acid (HNO3), perchloric acid (HClO4), and chloric acid (HClO3).
- The seven strong bases are sodium hydroxide (NaOH), potassium hydroxide (KOH), calcium hydroxide (Ca(OH)2), strontium hydroxide (Sr(OH)2), barium hydroxide (Ba(OH)2), lithium hydroxide (LiOH), and rubidium hydroxide (RbOH).
- Strong acids and bases are characterized by their complete dissociation in water, resulting in a high concentration of H+ or OH- ions.
- These substances are commonly used in various industrial and laboratory applications, including acid-base titrations, synthesis of compounds, and pH regulation.
- Understanding the properties and applications of strong acids and bases is essential for developing new technologies and addressing environmental challenges.
Properties of Strong Acids and Bases
Strong acids and bases are defined by their ability to completely dissociate in water, resulting in a high concentration of H+ or OH- ions. This complete dissociation is characterized by a high dissociation constant (Ka or Kb), which is a measure of the extent to which an acid or base dissociates in water. The seven strong acids have a Ka value greater than 1, indicating that they are highly acidic, while the seven strong bases have a Kb value greater than 1, indicating that they are highly basic.
Acid-Base Equilibrium
The acid-base equilibrium is a fundamental concept in chemistry, describing the balance between the concentrations of H+ and OH- ions in a solution. Strong acids and bases play a crucial role in this equilibrium, as they can significantly alter the pH of a solution. The pH scale, ranging from 0 to 14, is used to measure the acidity or basicity of a solution, with a pH of 7 being neutral. Strong acids and bases are often used to adjust the pH of a solution, making them essential in various industrial and laboratory applications.
| Strong Acid | Ka Value |
|---|---|
| Hydrochloric acid (HCl) | 1.3 x 10^6 |
| Hydrobromic acid (HBr) | 1.0 x 10^9 |
| Hydroiodic acid (HI) | 4.5 x 10^10 |
| Sulfuric acid (H2SO4) | 1.0 x 10^2 |
| Nitric acid (HNO3) | 2.4 x 10^1 |
| Perchloric acid (HClO4) | 1.0 x 10^10 |
| Chloric acid (HClO3) | 1.0 x 10^2 |
Applications of Strong Acids and Bases
Strong acids and bases have numerous applications in various fields, including industry, medicine, and environmental science. They are commonly used in acid-base titrations, synthesis of compounds, and pH regulation. For example, hydrochloric acid is used in the production of polyvinyl chloride (PVC), while sodium hydroxide is used in the manufacture of soap and paper. Additionally, strong acids and bases are used in various laboratory applications, including the preparation of buffers and the analysis of samples.
Environmental Significance
Strong acids and bases also play a crucial role in environmental science, particularly in the context of acid rain and water pollution. Acid rain, caused by the release of sulfuric acid and nitric acid into the atmosphere, can have devastating effects on ecosystems and infrastructure. Understanding the properties and applications of strong acids and bases is essential for developing strategies to mitigate the effects of acid rain and protect the environment.
| Strong Base | Kb Value |
|---|---|
| Sodium hydroxide (NaOH) | 1.0 x 10^14 |
| Potassium hydroxide (KOH) | 1.0 x 10^15 |
| Calcium hydroxide (Ca(OH)2) | 5.0 x 10^1 |
| Strontium hydroxide (Sr(OH)2) | 1.0 x 10^4 |
| Barium hydroxide (Ba(OH)2) | 1.0 x 10^3 |
| Lithium hydroxide (LiOH) | 1.0 x 10^2 |
| Rubidium hydroxide (RbOH) | 1.0 x 10^3 |
What is the difference between a strong acid and a weak acid?
+A strong acid is an acid that completely dissociates in water, resulting in a high concentration of H+ ions. A weak acid, on the other hand, only partially dissociates in water, resulting in a lower concentration of H+ ions.
What are some common applications of strong acids and bases?
+Strong acids and bases have numerous applications in various fields, including industry, medicine, and environmental science. They are commonly used in acid-base titrations, synthesis of compounds, and pH regulation.
How do strong acids and bases affect the environment?
+Strong acids and bases can have significant effects on the environment, particularly in the context of acid rain and water pollution. Understanding the properties and applications of strong acids and bases is essential for developing strategies to mitigate the effects of acid rain and protect the environment.
In conclusion, the seven strong acids and bases are essential substances in chemistry, characterized by their complete dissociation in water and high concentration of H+ or OH- ions. Understanding their properties, characteristics, and applications is crucial for various industrial and laboratory applications, as well as for addressing environmental challenges. By recognizing the significance of strong acids and bases, we can develop new technologies and strategies to mitigate their effects on the environment and promote sustainable development.



