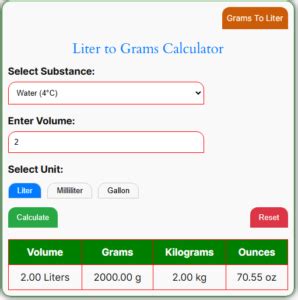
Converting liters to grams can be a bit tricky, as it requires knowing the density of the substance being measured. However, with a few simple steps and some basic knowledge of chemistry, you can easily make this conversion. In this article, we will explore five ways to convert liters to grams, using different methods and formulas.
Key Points
- Understanding the concept of density and its role in conversions
- Using the formula: mass = density × volume
- Applying different methods for various substances
- Converting between units using conversion factors
- Practicing with examples to reinforce understanding
Understanding Density and Conversion
Density is defined as mass per unit volume of a substance. It is typically expressed in units of grams per milliliter (g/mL) or kilograms per liter (kg/L). To convert liters to grams, we need to know the density of the substance. The formula to use is: mass = density × volume. For example, if we want to convert 2 liters of water to grams, we would use the density of water, which is approximately 1 g/mL or 1 kg/L.
Method 1: Using the Formula
Let’s use the formula to convert 2 liters of water to grams. First, we need to convert the volume from liters to milliliters, since the density is given in g/mL. There are 1000 milliliters in 1 liter, so 2 liters is equal to 2000 milliliters. Then, we can plug in the values: mass = density × volume = 1 g/mL × 2000 mL = 2000 grams.
| Substance | Density (g/mL) | Volume (mL) | Mass (g) |
|---|---|---|---|
| Water | 1 | 2000 | 2000 |
| Air | 0.0012 | 2000 | 2.4 |
Method 2: Using Conversion Factors

Another way to convert liters to grams is to use conversion factors. A conversion factor is a ratio of two units that are equal to each other. For example, we know that 1 liter is equal to 1000 milliliters, and 1 milliliter is equal to 1 gram. So, we can set up a conversion factor: 1 L = 1000 mL, and 1 mL = 1 g. Then, we can convert 2 liters to grams by multiplying by the conversion factors: 2 L × (1000 mL / 1 L) × (1 g / 1 mL) = 2000 g.
Method 3: Using a Calculator
For more complex conversions, it may be helpful to use a calculator. Many online calculators can convert units for you, including liters to grams. Simply enter the value you want to convert, select the units, and the calculator will do the rest. This can be especially useful for conversions involving multiple steps or complex formulas.
Method 4: Using a Conversion Chart
A conversion chart can also be a helpful tool for converting liters to grams. A conversion chart is a table that lists different units and their equivalent values. For example, a chart might list the density of different substances in g/mL, along with their equivalent values in kg/L. By looking up the density of the substance you are working with, you can easily convert liters to grams.
Method 5: Practicing with Examples
Finally, practicing with examples is one of the best ways to reinforce your understanding of conversions. Try converting different volumes of various substances to grams, using different methods and formulas. This will help you become more comfortable with the units and conversion factors, and you will be better able to apply your knowledge in real-world situations.
What is the density of water in g/mL?
+The density of water is approximately 1 g/mL.
How do I convert 5 liters of air to grams?
+First, look up the density of air in g/mL. Then, use the formula: mass = density × volume. Plug in the values and calculate the result.
What is the difference between a conversion factor and a formula?
+A conversion factor is a ratio of two units that are equal to each other, while a formula is a mathematical equation that describes a relationship between variables.
In conclusion, converting liters to grams requires an understanding of density and the ability to apply different methods and formulas. By practicing with examples and using conversion charts, calculators, and formulas, you can become more comfortable with these conversions and improve your overall understanding of chemistry and physics.