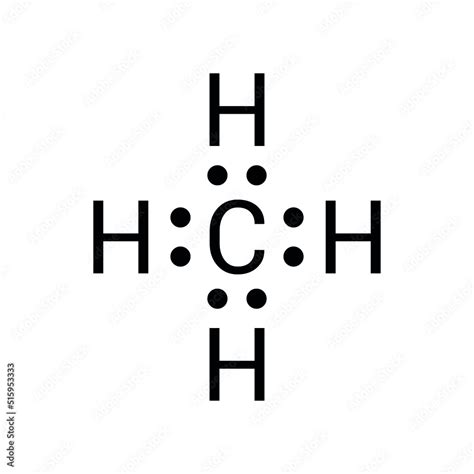
Methane, with the chemical formula CH4, is a simple yet fundamental molecule in organic chemistry. Understanding its structure is crucial for grasping more complex organic compounds. One of the primary ways to represent the structure of methane is through Lewis dot structures, which illustrate the distribution of electrons within the molecule. Here, we'll delve into how to draw a Lewis dot structure for methane and explore related concepts.
Introduction to Lewis Dot Structures
Lewis dot structures, also known as electron dot diagrams, are a graphical representation of the valence electrons of atoms in a molecule. They are a useful tool for predicting the shape of molecules and understanding chemical bonding. The process of drawing a Lewis dot structure involves several steps, including determining the total number of valence electrons, drawing single bonds between atoms, and then distributing the remaining electrons to satisfy the octet rule for each atom, where possible.
Step 1: Determine the Total Number of Valence Electrons
To draw the Lewis dot structure for methane (CH4), we start by calculating the total number of valence electrons. Carbon © has 4 valence electrons, and each hydrogen (H) has 1 valence electron. Since there are 4 hydrogen atoms, the total number of valence electrons for methane is 4 (from carbon) + 4*1 (from the four hydrogens) = 8 valence electrons.
Step 2: Draw Single Bonds Between Atoms
Next, we draw single bonds between the carbon atom and each of the four hydrogen atoms. Each single bond represents 2 shared electrons. With 4 single bonds, this accounts for 8 electrons (4 bonds * 2 electrons per bond). At this point, we’ve used all the valence electrons available in methane, and each atom has a full outer shell or octet, except for hydrogen, which is stable with 2 electrons.
Step 3: Confirm the Octet Rule
In the case of methane, after forming the four single bonds, we see that carbon has 8 electrons in its valence shell (4 bonds * 2 electrons per bond), and each hydrogen has 2 electrons, satisfying the duet rule for hydrogen. This configuration is stable and satisfies the octet rule for carbon, indicating a stable molecule.
| Atom | Valence Electrons | Shared Electrons in Methane |
|---|---|---|
| Carbon (C) | 4 | 8 (4 single bonds) |
| Hydrogen (H) | 1 | 2 (1 single bond each) |
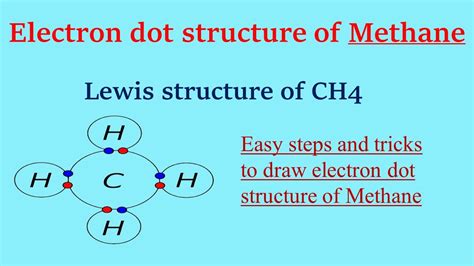
Importance of Lewis Dot Structures in Organic Chemistry
Lewis dot structures are more than just a tool for representing molecules; they are crucial for understanding chemical reactivity and bonding. For methane and other organic molecules, these structures help predict the polarity of bonds, the shape of molecules, and potential sites for chemical reactions. This knowledge is essential for designing and synthesizing new compounds with specific properties.
Limitations and Extensions
While Lewis dot structures provide valuable insights, they also have limitations. They do not account for the actual distribution of electrons in a molecule, which can be influenced by factors such as electronegativity differences between atoms. Furthermore, for larger molecules or those with delocalized electrons, more advanced methods such as resonance structures or molecular orbital theory may be necessary to fully understand their electronic structure.
Key Points
- The Lewis dot structure for methane involves a central carbon atom bonded to four hydrogen atoms, with no lone pairs on the carbon.
- Each bond in methane represents a pair of shared electrons, satisfying the octet rule for carbon and the duet rule for hydrogen.
- Understanding Lewis dot structures is crucial for predicting chemical properties and reactivity in organic chemistry.
- Lewis dot structures have limitations, particularly for molecules with delocalized electrons or significant electronegativity differences.
- Advanced methods like resonance structures and molecular orbital theory can provide a more complete understanding of molecular electronic structure.
As we explore the intricacies of organic chemistry, the humble methane molecule serves as a foundational example of how Lewis dot structures can illuminate our understanding of molecular bonding and reactivity. By mastering these concepts, chemists can design and synthesize new compounds with tailored properties, advancing fields from pharmaceuticals to materials science.
What is the significance of Lewis dot structures in organic chemistry?
+Lewis dot structures are significant in organic chemistry because they help predict the shape of molecules, the polarity of bonds, and potential sites for chemical reactions, which are essential for understanding chemical properties and designing new compounds.
How do you determine the total number of valence electrons in a molecule?
+The total number of valence electrons in a molecule is determined by adding the valence electrons of each atom in the molecule. For example, in methane (CH4), carbon has 4 valence electrons, and each hydrogen has 1, resulting in a total of 8 valence electrons.
What are the limitations of Lewis dot structures?
+Lewis dot structures have limitations, including not accounting for the actual distribution of electrons influenced by electronegativity differences and not being suitable for molecules with delocalized electrons. In such cases, more advanced methods like resonance structures or molecular orbital theory are necessary.