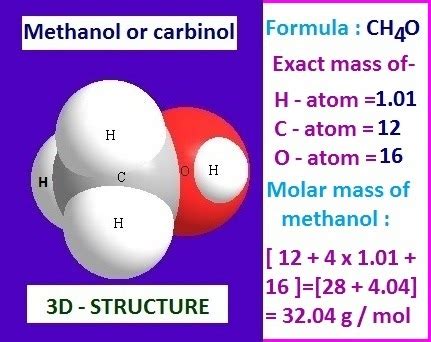
Methanol, also known as methyl alcohol or wood alcohol, is a chemical compound with the molecular formula CH₃OH. It is the simplest alcohol and is widely used as a solvent, antifreeze, and fuel. To understand the properties and behavior of methanol, it is essential to know its molecular weight, which is a fundamental physical property.
The molecular weight of methanol is calculated by summing the atomic weights of its constituent atoms. The atomic weight of carbon (C) is 12.01 g/mol, hydrogen (H) is 1.01 g/mol, and oxygen (O) is 16.00 g/mol. Therefore, the molecular weight of methanol can be calculated as follows: (1 x 12.01) + (4 x 1.01) + (1 x 16.00) = 12.01 + 4.04 + 16.00 = 32.05 g/mol. This means that one mole of methanol has a mass of 32.05 grams.
Key Points
- Methanol's molecular formula is CH₃OH, indicating one carbon, four hydrogens, and one oxygen atom.
- The atomic weights used for calculation are: Carbon = 12.01 g/mol, Hydrogen = 1.01 g/mol, Oxygen = 16.00 g/mol.
- The molecular weight of methanol is 32.05 g/mol, calculated by summing the atomic weights of its constituent atoms.
- Understanding methanol's molecular weight is crucial for its applications in chemistry, including as a solvent, fuel, and in the synthesis of other compounds.
- Methanol's properties, such as its boiling point (64.7°C) and melting point (-98°C), are also important for its industrial and laboratory uses.
Chemical Properties and Applications
Methanol’s chemical properties make it a versatile compound with a wide range of applications. Its ability to dissolve both polar and non-polar substances makes it an excellent solvent. Additionally, methanol is used as an antifreeze in windshield washer fluids and as a fuel in some vehicles, particularly those designed to run on alternative fuels. The production of formaldehyde, acetic acid, and methyl tertiary-butyl ether (MTBE) also relies heavily on methanol as a feedstock.
Production and Synthesis
Methanol is primarily produced through the catalytic hydrogenation of carbon monoxide and carbon dioxide, a process known as methanol synthesis. This reaction occurs over a copper-zinc oxide catalyst at high temperatures and pressures. The synthesis gas, a mixture of carbon monoxide, carbon dioxide, and hydrogen, is first produced from natural gas or coal, and then converted into methanol through the following reactions: CO + 2H₂ → CH₃OH and CO₂ + 3H₂ → CH₃OH + H₂O. These reactions are exothermic and require careful control of process conditions to optimize methanol yield and purity.
| Property | Value |
|---|---|
| Molecular Formula | CH₃OH |
| Molecular Weight | 32.05 g/mol |
| Boiling Point | 64.7°C |
| Melting Point | -98°C |
| Density | 0.7918 g/cm³ |
Environmental and Health Considerations
While methanol has numerous beneficial applications, its production, use, and disposal also raise environmental and health concerns. Methanol is toxic and can cause serious health effects if ingested, inhaled, or if it comes into contact with the skin. The environmental impact of methanol production is also significant, particularly if the synthesis gas is derived from non-renewable sources like natural gas or coal, contributing to greenhouse gas emissions. Additionally, the release of methanol into waterways can harm aquatic life, emphasizing the need for stringent regulations and safe handling practices.
Safety Measures and Regulations
To mitigate the risks associated with methanol, strict safety measures and regulations are in place. These include the use of personal protective equipment (PPE) when handling methanol, proper ventilation in workplaces where methanol is used, and adherence to spill response protocols. Regulatory bodies, such as the Occupational Safety and Health Administration (OSHA) in the United States, set permissible exposure limits (PELs) for methanol in the workplace, ensuring that workers are not exposed to harmful concentrations of the substance.
What is the primary method of methanol production?
+Methanol is primarily produced through the catalytic hydrogenation of carbon monoxide and carbon dioxide, known as methanol synthesis, using a copper-zinc oxide catalyst.
What are the main applications of methanol?
+Methanol is used as a solvent, antifreeze, fuel, and in the synthesis of formaldehyde, acetic acid, and methyl tertiary-butyl ether (MTBE), among other applications.
What are the environmental concerns related to methanol?
+The production of methanol from non-renewable sources contributes to greenhouse gas emissions, and its release into waterways can harm aquatic life, emphasizing the need for sustainable production methods and safe handling practices.
In conclusion, methanol’s molecular weight of 32.05 g/mol is a fundamental property that underpins its chemical behavior and applications. From its production through methanol synthesis to its use as a solvent, fuel, and feedstock for various chemical syntheses, understanding methanol’s properties is crucial for optimizing its benefits while minimizing its environmental and health impacts. As industries continue to evolve, the development of more sustainable methanol production methods and the exploration of its potential in emerging technologies will be essential for balancing economic, environmental, and social considerations.