The conversion of milliliters (mL) to grams (g) is a fundamental concept in various fields, including chemistry, physics, and everyday applications. However, this conversion is not as straightforward as it seems, because the density of the substance being measured plays a crucial role. In this article, we will explore five ways to convert mL to grams, considering different scenarios and substances.
Key Points
- Understanding the concept of density and its role in conversions between volume and mass.
- Using the formula: mass = density × volume, for precise conversions.
- Applying conversion factors for specific substances, such as water, where 1 mL equals 1 gram.
- Utilizing online conversion tools and calculators for quick and accurate conversions.
- Considering the importance of precise measurements in scientific and practical applications.
Understanding Density and Its Role in Conversion
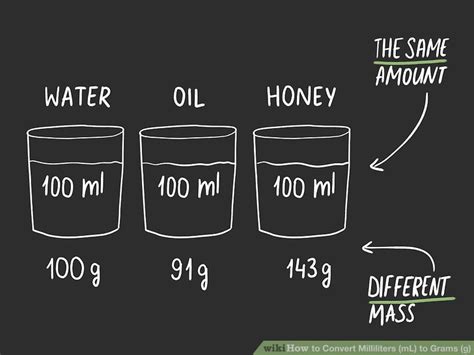
Density is defined as mass per unit volume of a substance. It is expressed in units such as grams per cubic centimeter (g/cm³) or grams per milliliter (g/mL). The density of a substance is crucial for converting between volume (in mL) and mass (in grams) because it tells us how much mass is contained in a given volume of the substance. For example, the density of water is approximately 1 g/mL, which means that 1 mL of water has a mass of 1 gram.
Converting mL to Grams Using Density
To convert mL to grams, we use the formula: mass (in grams) = density (in g/mL) × volume (in mL). For instance, if we want to find the mass of 500 mL of a substance with a density of 0.8 g/mL, we calculate it as follows: mass = 0.8 g/mL × 500 mL = 400 grams.
| Substance | Density (g/mL) |
|---|---|
| Water | 1.0 |
| Air | 0.0012 |
| Mercury | 13.546 |

Using Conversion Factors for Specific Substances
![[Solved] If A Patient Is Receiving 175 Ml Of 5.00% (M/V) Glucose ... [Solved] If A Patient Is Receiving 175 Ml Of 5.00% (M/V) Glucose ...](https://search.sks.com/assets/img/solved-if-a-patient-is-receiving-175-ml-of-5-00-m-v-glucose.jpeg)
For certain substances, especially water, a direct conversion factor can be used. Since the density of water is approximately 1 g/mL, 1 mL of water is equivalent to 1 gram. This makes conversions for water straightforward without needing to calculate density. However, it’s essential to remember that this 1:1 ratio is specific to water and does not apply universally across all substances.
Utilizing Online Conversion Tools
In today’s digital age, numerous online tools and calculators are available that can convert mL to grams for various substances. These tools often have built-in databases of densities for common substances, making the conversion process quick and easy. Users simply need to input the volume in mL and select the substance they are converting for, and the tool calculates the mass in grams.
Importance of Precise Measurements
Precise measurements are vital in many fields, including science, engineering, and cooking. In scientific research, accurate conversions between volume and mass are critical for experimental results and data analysis. Similarly, in cooking, the right balance of ingredients, which often involves precise measurements, can make a significant difference in the final product’s quality and taste.
Real-World Applications
Beyond laboratory settings, the conversion of mL to grams has numerous real-world applications. For example, in the manufacturing of food products, precise measurements are necessary to ensure consistency and compliance with nutritional labeling regulations. Additionally, in the pharmaceutical industry, accurate dosing depends on precise conversions between volumes and masses of active ingredients.
What is the density of air, and how does it affect conversions?
+The density of air is approximately 0.0012 g/mL. This means that for gases like air, the mass in grams for a given volume in mL is significantly less than for liquids or solids due to its much lower density.
Why is understanding density important for conversions?
+Understanding density is crucial because it allows for the accurate conversion between volume and mass. Different substances have different densities, and without this information, conversions cannot be accurately made.
Can I use online conversion tools for all types of substances?
+While online conversion tools are very useful, they may not cover all substances, especially less common ones. It's always a good idea to verify the density of the substance you are working with to ensure accurate conversions.
In conclusion, converting mL to grams is a process that requires understanding the concept of density and how it varies among different substances. Whether through manual calculations using the formula mass = density × volume, applying conversion factors for specific substances like water, or utilizing online conversion tools, accurate conversions are essential for both scientific and practical applications. By grasping the principles behind these conversions and applying them appropriately, individuals can ensure precision in their measurements, which is critical in a wide range of fields.