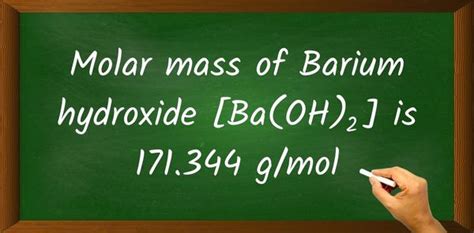Barium hydroxide, also known as barium hydroxide octahydrate, is a chemical compound with the molecular formula Ba(OH)₂. To calculate the molar mass of barium hydroxide, we need to sum the atomic masses of its constituent elements: barium (Ba), oxygen (O), and hydrogen (H). The atomic mass of barium is 137.327 g/mol, oxygen is 15.999 g/mol, and hydrogen is 1.008 g/mol.
Calculation of Molar Mass

The molar mass of barium hydroxide can be calculated as follows: Ba(OH)₂ = 1(Ba) + 2(O) + 2(H). Substituting the atomic masses, we get: molar mass = 137.327 g/mol + 2(15.999 g/mol) + 2(1.008 g/mol). Performing the calculation: molar mass = 137.327 g/mol + 31.998 g/mol + 2.016 g/mol = 171.341 g/mol.
Anhydrous vs. Hydrated Forms
It’s essential to note that barium hydroxide can exist in both anhydrous (Ba(OH)₂) and hydrated forms, such as barium hydroxide octahydrate (Ba(OH)₂·8H₂O). The molar mass calculated above refers to the anhydrous form. For the hydrated form, we need to account for the additional water molecules. The molar mass of water (H₂O) is 18.015 g/mol. For the octahydrate, the molar mass would be: 171.341 g/mol (anhydrous Ba(OH)₂) + 8(18.015 g/mol) (water) = 171.341 g/mol + 144.12 g/mol = 315.461 g/mol.
Key Points
- The molar mass of anhydrous barium hydroxide (Ba(OH)₂) is 171.341 g/mol.
- For barium hydroxide octahydrate (Ba(OH)₂·8H₂O), the molar mass is 315.461 g/mol, accounting for the additional water molecules.
- Understanding the molar mass of compounds is crucial for chemical calculations, such as preparing solutions or calculating reactant quantities in chemical reactions.
- The atomic masses used for calculations are approximate and based on the IUPAC standard atomic weights.
- Barium hydroxide is used in various applications, including the production of glass, rubber, and as a laboratory reagent.
The calculation of molar mass is a fundamental concept in chemistry, allowing for the determination of the mass of substances involved in chemical reactions. This is particularly important in quantitative chemistry, where precise measurements are required for experimental procedures. The molar mass of barium hydroxide, whether in its anhydrous or hydrated form, is a critical piece of information for chemists and researchers working with this compound.
Applications and Uses

Barium hydroxide has several applications due to its strong alkaline properties. It is used in the production of detergents, as a water softener, and in the manufacturing of glass and rubber. In laboratory settings, barium hydroxide is used as a strong base for various chemical reactions. The octahydrate form of barium hydroxide is more commonly used due to its easier handling and lower risk of causing skin and eye irritation compared to the anhydrous form.
Safety and Handling
Despite its usefulness, barium hydroxide requires careful handling due to its caustic nature. It can cause severe skin and eye irritation, and prolonged exposure can lead to more serious health issues. When working with barium hydroxide, it is essential to wear protective clothing, including gloves and goggles, and to work in a well-ventilated area. In the event of skin contact, the affected area should be flushed with water, and medical attention should be sought if irritation persists.
| Compound | Molar Mass (g/mol) |
|---|---|
| Barium (Ba) | 137.327 |
| Oxygen (O) | 15.999 |
| Hydrogen (H) | 1.008 |
| Barium Hydroxide (Ba(OH)₂) | 171.341 |
| Barium Hydroxide Octahydrate (Ba(OH)₂·8H₂O) | 315.461 |

In conclusion, understanding the molar mass of barium hydroxide, both in its anhydrous and hydrated forms, is essential for its safe and effective use in various chemical applications. By recognizing the importance of precise chemical calculations, chemists and researchers can better utilize barium hydroxide in their work, whether in industrial processes or laboratory experiments.
What is the molar mass of anhydrous barium hydroxide?
+The molar mass of anhydrous barium hydroxide (Ba(OH)₂) is 171.341 g/mol.
How does the molar mass change for barium hydroxide octahydrate?
+For barium hydroxide octahydrate (Ba(OH)₂·8H₂O), the molar mass is 315.461 g/mol, which includes the mass of the additional water molecules.
What are some common applications of barium hydroxide?
+Barium hydroxide is used in the production of glass, rubber, as a water softener, and as a laboratory reagent due to its strong alkaline properties.