The molar mass of a compound is the sum of the atomic masses of its constituent atoms. To calculate the molar mass of Fe2O3, also known as iron(III) oxide or ferric oxide, we need to know the atomic masses of iron (Fe) and oxygen (O). The atomic mass of iron is approximately 55.847 g/mol, and the atomic mass of oxygen is approximately 15.999 g/mol.
Calculation of Molar Mass
The formula Fe2O3 indicates that one molecule of iron(III) oxide contains 2 iron atoms and 3 oxygen atoms. Therefore, the molar mass of Fe2O3 can be calculated as follows:
Molar mass of Fe2O3 = (2 * atomic mass of Fe) + (3 * atomic mass of O)
Molar mass of Fe2O3 = (2 * 55.847 g/mol) + (3 * 15.999 g/mol)
Molar mass of Fe2O3 = 111.694 g/mol + 47.997 g/mol
Molar mass of Fe2O3 = 159.691 g/mol
Precision and Rounding
In calculations involving atomic masses, it’s essential to maintain precision until the final step to ensure accuracy. However, for most purposes, the molar mass of Fe2O3 can be rounded to a more manageable form. Rounding to the nearest whole number or to one decimal place is common, depending on the context of the calculation.
| Element | Atomic Mass (g/mol) | Number of Atoms in Fe2O3 | Total Mass Contribution (g/mol) |
|---|---|---|---|
| Iron (Fe) | 55.847 | 2 | 111.694 |
| Oxygen (O) | 15.999 | 3 | 47.997 |
| Total | 159.691 |
Key Points
- The atomic mass of iron (Fe) is approximately 55.847 g/mol.
- The atomic mass of oxygen (O) is approximately 15.999 g/mol.
- The molar mass of Fe2O3 is calculated by summing the masses of its constituent atoms: 2 iron atoms and 3 oxygen atoms.
- The calculated molar mass of Fe2O3 is 159.691 g/mol.
- Precision in calculation is crucial, but rounding may be applied based on the context of use.
Applications and Importance
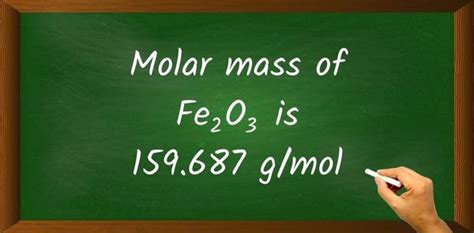
The molar mass of Fe2O3 has significant implications in various fields, including chemistry, materials science, and environmental science. Iron(III) oxide is a common compound found in nature as the mineral hematite and is used in numerous industrial applications, such as the production of iron and steel, catalysts, and pigments.
In chemical reactions, knowing the molar mass of reactants and products is essential for calculating stoichiometric ratios, which in turn affect the yield and efficiency of the reaction. Additionally, the molar mass of Fe2O3 is critical in the preparation of solutions for analytical purposes, where accurate concentrations are required.
Environmental Considerations
Fe2O3 also plays a role in environmental science, particularly in the study of soil chemistry and the fate of iron in aquatic ecosystems. Understanding the molar mass and subsequent chemical properties of iron(III) oxide can help in predicting its behavior and interactions with other substances in the environment.
What is the significance of knowing the molar mass of Fe2O3?
+Knowing the molar mass of Fe2O3 is crucial for various chemical calculations, including the preparation of solutions, calculating yields in reactions, and determining empirical formulas. It also has implications in industrial applications and environmental studies.
How is the molar mass of Fe2O3 calculated?
+The molar mass of Fe2O3 is calculated by summing the atomic masses of its constituent atoms: 2 atoms of iron (Fe) and 3 atoms of oxygen (O). The atomic masses are approximately 55.847 g/mol for Fe and 15.999 g/mol for O.
What are some of the industrial applications of Fe2O3?
+Fe2O3, or iron(III) oxide, is used in the production of iron and steel, as catalysts, and as pigments. Its applications are diverse due to its chemical and physical properties.
In conclusion, the molar mass of Fe2O3, calculated as 159.691 g/mol, is a fundamental piece of information with wide-ranging implications across various disciplines. Its accuracy is paramount for both theoretical calculations and practical applications, underscoring the importance of precise atomic mass values and careful calculation.