The concept of molar mass is a fundamental aspect of chemistry, serving as a bridge between the atomic and macroscopic levels of measurement. Molar mass, by definition, is the mass of one mole of a substance, where one mole is approximately 6.022 x 10^23 particles (atoms or molecules). Understanding molar mass is crucial for calculating the quantities of substances involved in chemical reactions, determining the composition of mixtures, and predicting the physical properties of compounds. In this article, we will explore six key ways molar mass impacts and is utilized in chemistry, highlighting its importance in both theoretical and practical applications.
Key Points
- Molar mass is essential for calculating the amount of substance in a given mass of a chemical compound.
- It plays a critical role in determining the empirical and molecular formulas of compounds.
- Molar mass is used in the calculation of the density of gases and the properties of solutions.
- Understanding molar mass is crucial for predicting the physical properties of compounds, such as boiling and melting points.
- It is vital in stoichiometry for balancing chemical equations and calculating the quantities of reactants and products.
- Molar mass is used in the preparation of solutions with specific concentrations, which is critical in laboratory settings.
Calculating Amount of Substance
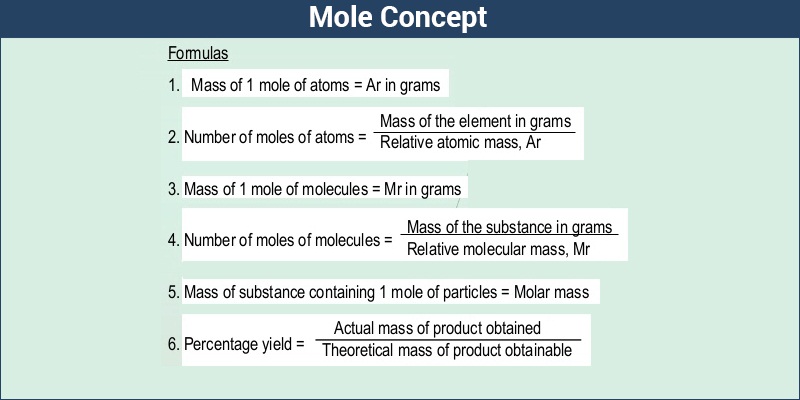
The molar mass of a substance is directly used to calculate the amount of substance (in moles) present in a given mass of that substance. This calculation is fundamental in chemistry and is expressed by the formula: amount of substance (n) = mass (m) / molar mass (M). For instance, to find out how many moles of sodium chloride (NaCl) are present in 100 grams of the substance, one would divide 100 grams by the molar mass of NaCl, which is approximately 58.44 grams per mole. This yields about 1.71 moles of NaCl.
Determining Empirical and Molecular Formulas
Molar mass is instrumental in determining the empirical and molecular formulas of compounds. The empirical formula is the simplest whole-number ratio of atoms of each element present in a compound, while the molecular formula shows the actual number of atoms of each element in a molecule. By comparing the molar mass of a compound to the mass of its empirical formula, one can determine if the empirical formula is also the molecular formula or if the molecular formula is a multiple of the empirical formula. For example, if the empirical formula of a compound has a mass of 60 grams per mole and the molar mass of the compound is 120 grams per mole, the molecular formula is twice the empirical formula.
Stoichiometry and Chemical Reactions

In the context of stoichiometry, molar mass is critical for balancing chemical equations and calculating the quantities of reactants and products involved in a reaction. By knowing the molar masses of the substances involved, chemists can calculate the limiting reagent, theoretical yields, and percent yields of reactions. This is essential in optimizing reaction conditions, predicting outcomes, and scaling up processes from the laboratory to industrial levels. For instance, in the synthesis of ammonia (NH3) from nitrogen (N2) and hydrogen (H2), the balanced equation is N2 + 3H2 → 2NH3. Knowing the molar masses allows for the calculation of how much NH3 can be produced from given amounts of N2 and H2.
Physical Properties and Solution Preparation
Molar mass can also provide insights into the physical properties of compounds, such as boiling and melting points, although these relationships are more complex and involve other factors like intermolecular forces. Additionally, molar mass is used in the preparation of solutions with specific concentrations, a process critical in laboratory settings for experiments, titrations, and other analyses. For example, to prepare a 1 molar (1 M) solution of sodium hydroxide (NaOH), one would dissolve the molar mass of NaOH (approximately 40.00 grams per mole) in enough water to make one liter of solution.
| Compound | Molar Mass (g/mol) | Empirical Formula | Molecular Formula |
|---|---|---|---|
| Sodium Chloride | 58.44 | NaCl | NaCl |
| Glucose | 180.16 | CH2O | C6H12O6 |
| Ammonia | 17.03 | NH3 | NH3 |

Conclusion and Future Directions
In conclusion, molar mass is a foundational concept in chemistry, underpinning various aspects of chemical theory and practice. From calculating the amount of substance in a given mass to determining empirical and molecular formulas, and from stoichiometry to the preparation of solutions, molar mass plays a pivotal role. As chemistry continues to evolve, with advancements in fields like materials science, biochemistry, and environmental chemistry, the importance of molar mass will only continue to grow. Future research and applications will likely rely heavily on precise calculations and understandings of molar masses, especially as scientists delve into the complexities of nanotechnology, pharmaceutical development, and climate science.
What is the significance of molar mass in chemical reactions?
+Molar mass is crucial in chemical reactions for calculating the quantities of reactants and products, determining the limiting reagent, and predicting the outcomes of reactions.
How is molar mass used in determining the empirical and molecular formulas of compounds?
+Molar mass helps in determining if the empirical formula of a compound is also its molecular formula or if the molecular formula is a multiple of the empirical formula by comparing their masses.
What role does molar mass play in the preparation of solutions?
+Molar mass is essential for preparing solutions of specific concentrations by determining how much of a substance is needed to achieve a certain molarity.