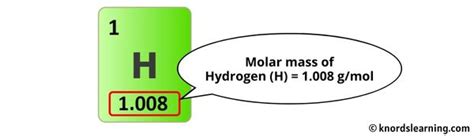
The concept of molar mass is fundamental in chemistry, representing the mass of one mole of a substance. For hydrogen gas, denoted as H2, understanding its molar mass is crucial for various chemical calculations and reactions. The molar mass of H2 can be determined through several methods, each reflecting different aspects of chemical principles. Here, we'll explore five ways to approach the calculation of the molar mass of H2, highlighting the underlying chemistry and the importance of precise calculations in chemical studies.
Understanding Molar Mass Basics

Molar mass is defined as the mass of one mole of a substance. It is expressed in units of grams per mole (g/mol). For elements, the molar mass is the sum of the atomic masses of the atoms in one molecule of the element, considering the natural isotopic abundance. The atomic mass of hydrogen is approximately 1.00794 u (unified atomic mass units), where u is defined as one-twelfth the mass of a carbon-12 atom.
Method 1: Direct Calculation from Atomic Mass
The most straightforward method to calculate the molar mass of H2 is by summing the atomic masses of two hydrogen atoms, since a molecule of hydrogen gas consists of two hydrogen atoms. The calculation is as follows: Molar mass of H2 = 2 * atomic mass of H = 2 * 1.00794 u = 2.01588 u. To convert this to grams per mole, we use the fact that 1 u = 1 g/mol, so the molar mass of H2 is approximately 2.01588 g/mol.
| Element | Atomic Mass (u) | Molar Mass Contribution (g/mol) |
|---|---|---|
| Hydrogen (H) | 1.00794 | 1.00794 g/mol |
| Hydrogen (H) | 1.00794 | 1.00794 g/mol |
| Total for H2 | 2.01588 | 2.01588 g/mol |
Exploring Isotopic Variations
Beyond the standard calculation, considering isotopic variations can provide a deeper understanding of the molar mass concept. Deuterium, for example, has an atomic mass of approximately 2.0141 u. If we were to calculate the molar mass of a molecule of hydrogen consisting of one protium and one deuterium (HD), the calculation would be: Molar mass of HD = atomic mass of H + atomic mass of D = 1.00794 u + 2.0141 u = 3.02204 u, or approximately 3.02204 g/mol.
Method 2: Using the Periodic Table
The periodic table provides atomic masses that are averages reflecting the natural abundance of isotopes. For hydrogen, the standard atomic weight is listed as 1.00794 u. Thus, the molar mass of H2 can be directly calculated as twice this value, accounting for the diatomic nature of hydrogen gas.
Method 3: Empirical Formula and Molecular Weight
In cases where the molecular formula is known, the empirical formula can be used to calculate the molar mass. For H2, the empirical and molecular formulas are the same. The molecular weight (or formula weight) for H2, considering the atomic weights, is simply the sum of the atomic weights of the atoms in the formula unit.
Method 4: From Density and Molar Volume
Another approach involves using the density of hydrogen gas and the molar volume of an ideal gas. The molar volume of an ideal gas at standard temperature and pressure (STP) is approximately 22.4 liters. If the density of hydrogen gas at STP is known, the molar mass can be calculated using the formula: Molar mass = density * molar volume. This method, while less direct, illustrates the relationship between physical properties and molar mass.
Method 5: Experimental Determination
Experimentally, the molar mass of a gas can be determined using techniques such as measuring the mass of a known volume of the gas at a specified temperature and pressure, and then applying the ideal gas law (PV = nRT) to find the number of moles, from which the molar mass can be calculated. This method is more complex and subject to experimental errors but provides a practical application of chemical principles.
Key Points
- The molar mass of H2 is approximately 2.01588 g/mol, calculated from the atomic masses of hydrogen.
- Isotopic variations, such as deuterium, can significantly affect the molar mass of hydrogen isotopologues.
- The periodic table provides a quick reference for atomic masses to calculate molar masses of elements and compounds.
- Empirical and molecular formulas are essential for calculating molar masses, especially for compounds.
- Physical properties like density and molar volume can be used to determine molar mass through experimental methods.
In conclusion, calculating the molar mass of H2 involves understanding the atomic mass of hydrogen, the diatomic nature of hydrogen gas, and applying basic chemical principles. Whether through direct calculation, consideration of isotopic variations, or experimental determination, each method underscores the importance of precision and the interplay between chemical concepts and physical properties.
What is the primary factor affecting the molar mass of hydrogen gas?
+The primary factor is the atomic mass of hydrogen itself, considering its natural isotopic abundance.
How does the presence of deuterium affect the molar mass of hydrogen?
+Deuterium, being approximately twice as heavy as protium, significantly increases the molar mass of any hydrogen molecule it is part of, such as HD.
What role does the periodic table play in calculating molar masses?
+The periodic table provides the standard atomic weights of elements, which are used to calculate the molar masses of elements and compounds.