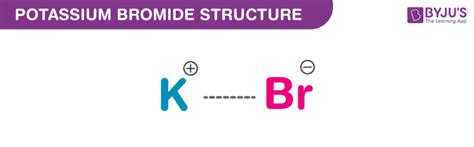
The molar mass of a compound is a fundamental concept in chemistry, representing the mass of one mole of that substance. For potassium bromide (KBr), a chemical compound consisting of one potassium (K) atom and one bromine (B) atom, calculating the molar mass involves summing the atomic masses of its constituent atoms. The atomic mass of potassium (K) is approximately 39.0983 grams per mole, and the atomic mass of bromine (Br) is about 79.904 grams per mole.
Calculation of Molar Mass of KBr

To calculate the molar mass of KBr, we add the atomic masses of potassium and bromine. The formula for this calculation is: Molar Mass of KBr = Atomic Mass of K + Atomic Mass of Br. Substituting the known values, we get Molar Mass of KBr = 39.0983 g/mol + 79.904 g/mol.
Performing the Calculation
Adding these values together, Molar Mass of KBr = 39.0983 g/mol + 79.904 g/mol = 119.0023 g/mol. Therefore, the molar mass of potassium bromide (KBr) is approximately 119.00 g/mol when rounded to the nearest whole number, which is a common practice for many chemical calculations.
| Element | Atomic Mass (g/mol) |
|---|---|
| Potassium (K) | 39.0983 |
| Bromine (Br) | 79.904 |
| Molar Mass of KBr | 119.0023 |
Key Points
- The molar mass of KBr is calculated by adding the atomic masses of potassium and bromine.
- The atomic mass of potassium is approximately 39.0983 g/mol.
- The atomic mass of bromine is about 79.904 g/mol.
- The molar mass of KBr is approximately 119.0023 g/mol.
- Molar mass is essential for quantitative chemical calculations and reactions.
Applications and Importance

The knowledge of the molar mass of KBr has various applications in chemistry, including the preparation of solutions, calculation of the amount of substance in a given mass of the compound, and determining the empirical and molecular formulas of compounds. In laboratory settings, molar mass is used to calculate the number of moles of a substance, which is crucial for stoichiometric calculations in chemical reactions.
Stoichiometry and Chemical Reactions
In the context of chemical reactions, knowing the molar mass of reactants and products is essential for calculating the limiting reactant, the yield of products, and the percentage yield of a reaction. For reactions involving KBr, such as its use as a source of bromide ions in synthesis reactions, understanding its molar mass facilitates the accurate measurement of reactants, ensuring that reactions are carried out efficiently and safely.
Furthermore, the molar mass of compounds like KBr plays a critical role in analytical chemistry, particularly in techniques such as gravimetric analysis, where the mass of a compound is used to determine its concentration or the concentration of other substances in a mixture. The precision in calculating molar masses and the subsequent calculations in chemical reactions underscores the importance of accurate atomic mass values for elements.
What is the significance of molar mass in chemical reactions?
+The molar mass is significant in chemical reactions as it allows for the calculation of the number of moles of a substance, which is crucial for determining the limiting reactant, the yield of products, and the stoichiometry of the reaction.
How is the molar mass of a compound calculated?
+The molar mass of a compound is calculated by summing the atomic masses of its constituent atoms. For KBr, this involves adding the atomic mass of potassium to the atomic mass of bromine.
What are the applications of knowing the molar mass of KBr?
+Knowing the molar mass of KBr has applications in preparing solutions, calculating the amount of substance in a given mass, determining empirical and molecular formulas, and in stoichiometric calculations for chemical reactions.
In conclusion, the molar mass of potassium bromide (KBr) is a fundamental piece of information in chemistry, with its calculation and application spanning various aspects of chemical analysis and reaction stoichiometry. The precision and accuracy of such calculations are paramount, underscoring the importance of up-to-date atomic mass values for elements and the careful application of these values in chemical computations.