The concept of molar mass is fundamental in chemistry, representing the mass of one mole of a substance. When considering the molar mass of potassium iodide (KI), it's essential to understand the atomic masses of its constituent elements, potassium (K) and iodine (I). The atomic mass of potassium is approximately 39.0983 g/mol, and the atomic mass of iodine is about 126.90447 g/mol. To calculate the molar mass of KI, we simply add the atomic masses of potassium and iodine.
Calculating Molar Mass of KI
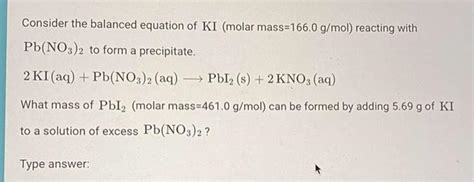
The calculation for the molar mass of KI is straightforward: Molar mass of KI = Atomic mass of K + Atomic mass of I. Substituting the known values, we get Molar mass of KI = 39.0983 g/mol + 126.90447 g/mol.
Performing the Calculation
By adding the atomic masses of potassium and iodine, we find that the molar mass of KI is approximately 166.00277 g/mol. This value represents the mass of one mole of potassium iodide and is crucial for various chemical calculations, including the preparation of solutions and the determination of reaction yields.
| Element | Atomic Mass (g/mol) |
|---|---|
| Potassium (K) | 39.0983 |
| Iodine (I) | 126.90447 |
| Potassium Iodide (KI) | 166.00277 |
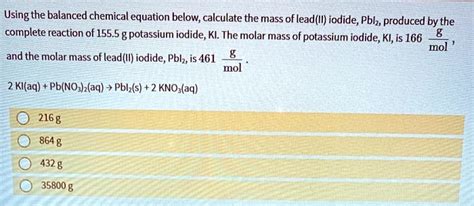
Practical Applications of Molar Mass

The molar mass of KI has several practical applications. For instance, in the preparation of iodine solutions for laboratory use, knowing the molar mass of KI enables the accurate calculation of how much KI is needed to achieve a specific concentration of iodine in the solution.
Importance in Stoichiometry
In chemical reactions involving KI, the molar mass is essential for determining the stoichiometric ratios of reactants and products. This ensures that reactions are carried out with the appropriate amounts of substances, which is critical for achieving the desired products and minimizing waste.
Key Points
- The molar mass of potassium iodide (KI) is calculated by adding the atomic masses of potassium (K) and iodine (I).
- The atomic mass of potassium is approximately 39.0983 g/mol, and the atomic mass of iodine is about 126.90447 g/mol.
- The molar mass of KI is crucial for various chemical calculations, including the preparation of solutions and the determination of reaction yields.
- Understanding the molar mass of compounds like KI is essential for stoichiometric calculations in chemistry.
- The molar mass of a substance is a fundamental property that connects the mass of a substance to the number of particles (atoms or molecules) it contains.
The calculation and understanding of the molar mass of substances like potassium iodide underscore the importance of precise measurements and calculations in chemistry. By grasping these fundamental concepts, chemists can design and execute experiments with accuracy, leading to advancements in various fields of science and technology.
What is the significance of molar mass in chemical reactions?
+The molar mass is significant because it allows chemists to calculate the number of moles of a substance, which is crucial for determining the stoichiometry of chemical reactions and preparing solutions of known concentration.
How is the molar mass of a compound calculated?
+The molar mass of a compound is calculated by summing the atomic masses of its constituent atoms. For potassium iodide (KI), it is the sum of the atomic masses of potassium and iodine.
What is the molar mass of potassium iodide (KI)?
+The molar mass of KI is approximately 166.00277 g/mol, calculated by adding the atomic mass of potassium (39.0983 g/mol) to the atomic mass of iodine (126.90447 g/mol).