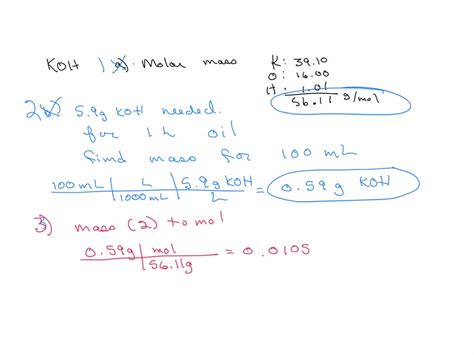KOH, or potassium hydroxide, is a strong base commonly used in various industrial and laboratory applications. Understanding its molar mass is crucial for calculating the amounts needed for reactions, solutions, and other processes. The molar mass of KOH can be calculated by summing the atomic masses of its constituent elements: potassium (K), oxygen (O), and hydrogen (H). Here, we'll explore the concept of molar mass, how it's calculated for KOH, and provide five different perspectives or methods through which the molar mass of KOH can be understood or applied.
Key Points
- The molar mass of KOH is calculated by adding the atomic masses of potassium, oxygen, and hydrogen.
- Understanding the molar mass of KOH is essential for chemical reactions and solution preparations.
- KOH is a strong base used in various applications, including soap making, battery production, and as a catalyst in chemical reactions.
- The calculation of KOH's molar mass involves precise atomic masses from the periodic table.
- Accurate molar mass calculations are critical for achieving desired outcomes in chemical processes.
Calculating the Molar Mass of KOH

The atomic masses (rounded to the nearest whole number for simplicity) are approximately: potassium (K) = 39 g/mol, oxygen (O) = 16 g/mol, and hydrogen (H) = 1 g/mol. Therefore, the molar mass of KOH can be calculated as follows: Molar mass of KOH = atomic mass of K + atomic mass of O + atomic mass of H = 39 g/mol + 16 g/mol + 1 g/mol = 56 g/mol.
Understanding the Role of KOH in Chemical Reactions
KOH plays a significant role in various chemical reactions, acting as a strong base. Its reactivity is determined by its ability to donate hydroxide ions (OH^-), which can neutralize acids and participate in other reactions. The molar mass of KOH is essential in these applications for determining the correct proportions of reactants.
| Element | Atomic Mass (g/mol) |
|---|---|
| Potassium (K) | 39 |
| Oxygen (O) | 16 |
| Hydrogen (H) | 1 |
| Molar Mass of KOH | 56 |

Applications of KOH and the Importance of Molar Mass

The applications of KOH are diverse, ranging from the production of soaps and detergents to its use in alkaline batteries and as a catalyst in chemical synthesis. In each of these applications, knowing the exact molar mass of KOH is vital for ensuring the correct chemical reactions occur, which directly impacts the quality and yield of the final products.
Implications of Accurate Molar Mass Calculations
Accurate calculations of the molar mass of KOH have significant implications for both the efficiency and safety of chemical processes. Incorrect proportions can lead to unwanted side reactions, reduced yields, and even pose safety risks due to the handling of reactive substances. Therefore, understanding and accurately calculating the molar mass of KOH is a fundamental skill in chemistry and chemical engineering.
As the chemical industry continues to evolve, with advancements in technology and the development of new materials and processes, the importance of precise chemical calculations, including the determination of molar masses, will only continue to grow. This underscores the need for professionals in the field to have a deep understanding of chemical principles and the ability to apply them accurately in real-world scenarios.
What is the molar mass of KOH used for in chemical reactions?
+The molar mass of KOH is used to calculate the amounts of KOH needed in chemical reactions to achieve the desired outcomes, ensuring the correct proportions of reactants are used.
How does the atomic mass of elements affect the molar mass of KOH?
+The atomic masses of potassium, oxygen, and hydrogen are summed to calculate the molar mass of KOH. Any variation in these atomic masses directly affects the calculated molar mass of KOH.
What are some common applications of KOH where its molar mass is critical?
+KOH is used in soap making, battery production, and as a catalyst in chemical reactions. In these applications, the molar mass of KOH is crucial for determining the correct amounts to use for the desired chemical reactions and product yields.
In conclusion, the molar mass of KOH, calculated by summing the atomic masses of its constituent elements, is a fundamental piece of information in chemistry and chemical engineering. Its applications are diverse, and understanding its molar mass is essential for achieving the desired outcomes in various chemical processes and reactions. As the field continues to evolve, the importance of accurate chemical calculations, including the determination of molar masses, will remain a critical component of professional practice.