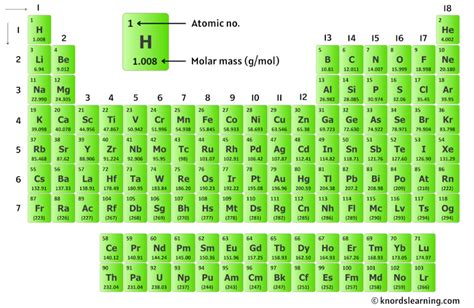
The calculation of the molar mass of N2, or nitrogen gas, is a fundamental concept in chemistry, reflecting the sum of the atomic masses of the two nitrogen atoms that make up a molecule of nitrogen. Understanding molar mass is crucial for quantifying the amount of substance in chemical reactions, especially when dealing with gases like nitrogen. Here, we will explore five ways to calculate or understand the molar mass of N2, each approach highlighting a different aspect of chemical calculation and principle.
Understanding Atomic Mass of Nitrogen
The atomic mass of nitrogen is approximately 14.01 g/mol. This value is an average of the masses of the naturally occurring isotopes of nitrogen, weighted by their abundance. The most common isotopes are nitrogen-14 (~99.6% abundance) and nitrogen-15 (~0.4% abundance). To calculate the molar mass of N2, we simply multiply the atomic mass of nitrogen by 2, since a molecule of nitrogen gas consists of two nitrogen atoms.
Direct Calculation
The direct calculation of N2’s molar mass involves multiplying the atomic mass of nitrogen by 2:
| Atomic Mass of Nitrogen | Number of Atoms in N2 | Molar Mass of N2 |
|---|---|---|
| 14.01 g/mol | 2 | 28.02 g/mol |

This straightforward calculation provides the molar mass of nitrogen gas as approximately 28.02 g/mol.
Key Points
- The atomic mass of nitrogen is approximately 14.01 g/mol.
- N2 consists of two nitrogen atoms.
- The molar mass of N2 is calculated by doubling the atomic mass of nitrogen.
- The molar mass of N2 is approximately 28.02 g/mol.
- Understanding molar mass is essential for chemical calculations and reactions.
Chemical Reactions and Stoichiometry
In chemical reactions, the molar mass of reactants and products is crucial for determining the quantities of substances involved. For nitrogen, its reaction with hydrogen to form ammonia (NH3) is a classic example. The balanced chemical equation for this reaction is N2 + 3H2 → 2NH3. Knowing the molar mass of N2 allows us to calculate the mass of nitrogen required to react with a given mass of hydrogen, based on the stoichiometry of the reaction.
Practical Application in Industry
The production of ammonia is a significant industrial process, where nitrogen from the air is fixed into a usable form. The molar mass of N2 is essential in calculating the efficiency and yield of this process, ensuring that the correct proportions of nitrogen and hydrogen are used to maximize ammonia production while minimizing waste and energy consumption.
The calculation of molar mass is also critical in understanding and predicting the behavior of gases, such as nitrogen, under various conditions. The ideal gas law, PV = nRT, where n is the number of moles, relies on the molar mass of the gas to convert between mass and moles. For N2, knowing its molar mass allows us to calculate the number of moles of nitrogen in a given volume at specific temperature and pressure conditions, which is vital in many industrial and laboratory settings.
Molecular Weight and Isotopic Variation
While the average atomic mass of nitrogen is used for most calculations, the actual molecular weight of N2 can vary slightly due to the presence of different isotopes. For example, a molecule of N2 could consist of two nitrogen-14 atoms, or a combination of nitrogen-14 and nitrogen-15. These variations, though minimal, can affect the precise molecular weight of N2 in specific samples. However, for most chemical and industrial applications, the average molar mass of 28.02 g/mol is sufficiently accurate.
Advanced Calculations and Considerations
In advanced chemical and physical analyses, the precise calculation of molar masses, including considerations for isotopic variations, becomes crucial. For instance, in mass spectrometry, the exact mass of molecules, including isotopic variants, is measured to identify and quantify substances. Understanding the molar mass of N2 and its potential variations is fundamental in interpreting such data and in applications where the distinction between different isotopic forms of nitrogen is significant.
What is the molar mass of N2?
+The molar mass of N2, or nitrogen gas, is approximately 28.02 g/mol, calculated by doubling the atomic mass of nitrogen (14.01 g/mol).
Why is understanding the molar mass of N2 important?
+Understanding the molar mass of N2 is crucial for chemical calculations, reactions, and industrial processes, especially in the production of ammonia and in understanding the behavior of gases.
How does isotopic variation affect the molar mass of N2?
+Isotopic variation can cause minimal variations in the molecular weight of N2, but for most applications, the average molar mass of 28.02 g/mol is used and considered sufficiently accurate.
In conclusion, the calculation and understanding of the molar mass of N2 are foundational aspects of chemistry, with applications ranging from basic stoichiometric calculations to advanced industrial processes and isotopic analyses. By grasping the concept of molar mass and its implications, one can better appreciate the intricacies of chemical reactions and the importance of precise measurements in scientific inquiry and industrial production.