The calculation of the molar mass of sodium phosphate, Na3PO4, is a fundamental concept in chemistry, particularly in the fields of inorganic chemistry and chemical analysis. To determine the molar mass of Na3PO4, we need to consider the atomic masses of its constituent elements: sodium (Na), phosphorus (P), and oxygen (O). The atomic masses of these elements are approximately sodium (Na) = 22.99 g/mol, phosphorus (P) = 30.97 g/mol, and oxygen (O) = 16.00 g/mol.
Understanding the Formula and Atomic Masses
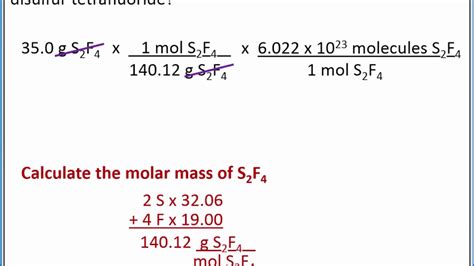
The formula Na3PO4 indicates that one molecule of sodium phosphate consists of three sodium atoms, one phosphorus atom, and four oxygen atoms. To calculate the molar mass, we sum the atomic masses of these atoms in the proportions given by the formula.
Calculating the Molar Mass of Na3PO4
The calculation involves multiplying the atomic mass of each element by the number of atoms of that element in the formula and then summing these values. For sodium, we have 3 atoms, so 3 * 22.99 g/mol. For phosphorus, there is 1 atom, so 1 * 30.97 g/mol. For oxygen, there are 4 atoms, so 4 * 16.00 g/mol. Adding these together gives us the molar mass of Na3PO4.
| Element | Atomic Mass (g/mol) | Number of Atoms | Total Mass Contribution (g/mol) |
|---|---|---|---|
| Sodium (Na) | 22.99 | 3 | 3 * 22.99 = 68.97 |
| Phosphorus (P) | 30.97 | 1 | 1 * 30.97 = 30.97 |
| Oxygen (O) | 16.00 | 4 | 4 * 16.00 = 64.00 |

Summing the total mass contributions from sodium, phosphorus, and oxygen gives us 68.97 g/mol + 30.97 g/mol + 64.00 g/mol = 163.94 g/mol. Therefore, the molar mass of Na3PO4 is approximately 163.94 g/mol.
Key Points
- The molar mass of Na3PO4 is calculated by summing the atomic masses of its constituent elements: sodium, phosphorus, and oxygen.
- The atomic masses used are approximately 22.99 g/mol for sodium, 30.97 g/mol for phosphorus, and 16.00 g/mol for oxygen.
- The formula Na3PO4 indicates three sodium atoms, one phosphorus atom, and four oxygen atoms, which are used to calculate the total mass contribution of each element.
- The total molar mass of Na3PO4 is approximately 163.94 g/mol, calculated as (3*22.99) + (1*30.97) + (4*16.00).
- Understanding and calculating molar mass is essential for chemical reactions, stoichiometric calculations, and chemical analysis.
Practical Applications and Considerations
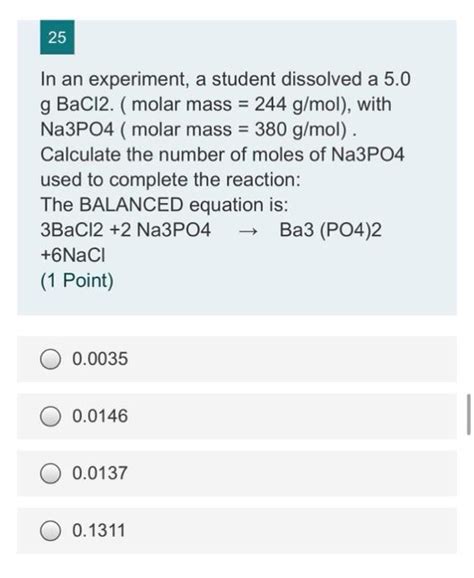
The molar mass of Na3PO4 has practical implications in various fields, including chemistry, biology, and environmental science. In laboratory settings, knowing the molar mass of compounds is essential for preparing solutions, calculating reactant quantities, and understanding reaction stoichiometry. Additionally, the molar mass of compounds like Na3PO4 is critical in industrial processes, such as the manufacture of detergents, water treatment chemicals, and fertilizers, where precise control over chemical quantities is necessary.
Chemical Reactions and Stoichiometry
In chemical reactions involving Na3PO4, the molar mass is used to calculate the number of moles of reactants and products, based on the given masses or volumes. This is fundamental to understanding reaction stoichiometry, predicting yields, and optimizing reaction conditions. For instance, in a reaction where Na3PO4 is used as a reactant, knowing its molar mass allows chemists to calculate the exact amount needed to react completely with other reactants, minimizing waste and maximizing efficiency.
Moreover, the molar mass of Na3PO4 is essential in analytical chemistry for quantifying the amount of substance in a sample. Techniques such as gravimetric analysis, where the mass of a compound is measured after precipitation or reaction, rely on accurate molar mass values to calculate the number of moles and, consequently, the concentration of the analyte in the sample.
What is the primary use of molar mass in chemistry?
+The primary use of molar mass in chemistry is for calculating the number of moles of a substance, which is crucial for stoichiometric calculations, preparing solutions, and understanding chemical reactions.
How does the molar mass of Na3PO4 affect its industrial applications?
+The molar mass of Na3PO4 is critical in its industrial applications, such as in the production of detergents and fertilizers, where precise quantities of reactants are necessary to achieve the desired product composition and minimize costs.
What role does the molar mass of Na3PO4 play in environmental science?
+In environmental science, the molar mass of Na3PO4 is relevant in studies of water pollution, where phosphate levels can indicate the degree of eutrophication. Accurate molar mass values help in quantifying phosphate concentrations and understanding their ecological impact.
In conclusion, the molar mass of Na3PO4, calculated as approximately 163.94 g/mol, is a fundamental property with significant implications in chemical reactions, industrial processes, and environmental studies. Understanding and accurately calculating molar masses are essential skills in chemistry, facilitating precise measurements, stoichiometric calculations, and the prediction of reaction outcomes.