The concept of NaHCO3, commonly known as sodium bicarbonate or baking soda, is widely recognized for its versatile applications in various industries, including food, pharmaceuticals, and chemicals. The mass of NaHCO3 can be determined and utilized in different ways, depending on the specific requirements of the application. Here, we will explore three distinct methods to calculate or apply the mass of NaHCO3, highlighting their relevance and importance in different contexts.
Understanding NaHCO3 and Its Applications
NaHCO3 is a white solid that is highly soluble in water, making it a valuable compound in numerous chemical reactions and processes. Its applications range from use as a leavening agent in baked goods to its role in pharmaceutical formulations. The mass of NaHCO3 is crucial in these applications, as precise measurements are necessary to achieve the desired outcomes. For instance, in the food industry, the mass of NaHCO3 used can affect the texture and taste of the final product.
Method 1: Calculating Molar Mass
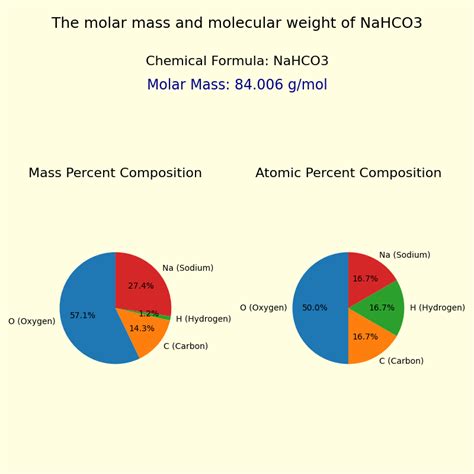
The molar mass of NaHCO3 can be calculated by summing the atomic masses of its constituent elements: sodium (Na), hydrogen (H), carbon ©, and oxygen (O). The atomic masses (rounded to the nearest whole number for simplicity) are approximately: Na = 23 g/mol, H = 1 g/mol, C = 12 g/mol, and O = 16 g/mol. Since there are three oxygen atoms in the formula, the total mass contributed by oxygen is 3 * 16 = 48 g/mol. Thus, the molar mass of NaHCO3 is 23 (Na) + 1 (H) + 12 © + 48 (O) = 84 g/mol.
| Element | Atomic Mass (g/mol) |
|---|---|
| Sodium (Na) | 23 |
| Hydrogen (H) | 1 |
| Carbon (C) | 12 |
| Oxygen (O) | 16 |
Practical Applications of NaHCO3 Mass
Beyond the calculation of molar mass, the actual mass of NaHCO3 used in various applications can significantly impact the outcome. For example, in cooking, the mass of baking soda used can affect the rise of dough or the texture of cookies. Similarly, in pharmaceutical applications, precise control over the mass of NaHCO3 is necessary to ensure the efficacy and safety of drug formulations.
Method 2: Measurement for Cooking and Baking
In culinary applications, the mass of NaHCO3 is crucial for achieving the right chemical reaction, which affects the final product’s texture and taste. A common method for measuring the mass of NaHCO3 in cooking is by using a digital kitchen scale, which provides accurate measurements in grams. For instance, a recipe might call for 5 grams of baking soda to leaven a specific quantity of dough.
Method 3: Industrial and Pharmaceutical Applications
In industrial and pharmaceutical contexts, the mass of NaHCO3 must be measured with high precision. This is often achieved using analytical balances that can measure masses with an accuracy of 0.1 milligrams or better. The precise control over the mass of NaHCO3 is critical in these applications, as it directly impacts the quality and efficacy of the final product.
Key Points
- The molar mass of NaHCO3 is calculated as 84 g/mol by summing the atomic masses of its constituent elements.
- The actual mass of NaHCO3 used in applications can significantly affect the outcome, whether in cooking, pharmaceutical formulations, or industrial processes.
- Accurate measurement of NaHCO3 mass is critical, with methods ranging from digital kitchen scales for cooking to analytical balances for industrial and pharmaceutical applications.
- Understanding and controlling the mass of NaHCO3 is essential for achieving desired chemical reactions and product qualities.
- Precision in measuring NaHCO3 mass is a reflection of the broader importance of quantitative analysis in chemistry and related fields.
In conclusion, the mass of NaHCO3, whether calculated as molar mass or measured for specific applications, plays a vital role in various industries and everyday life. The precision and accuracy in determining and utilizing the mass of NaHCO3 underscore the importance of quantitative analysis and attention to detail in achieving desired outcomes.
What is the molar mass of NaHCO3?
+The molar mass of NaHCO3 is 84 g/mol, calculated by summing the atomic masses of sodium, hydrogen, carbon, and oxygen.
Why is the mass of NaHCO3 important in cooking?
+The mass of NaHCO3 is crucial in cooking as it affects the chemical reaction that causes dough to rise or changes the texture of baked goods.
How is the mass of NaHCO3 measured in industrial applications?
+In industrial applications, the mass of NaHCO3 is typically measured using analytical balances that provide high precision, often to 0.1 milligrams or better.