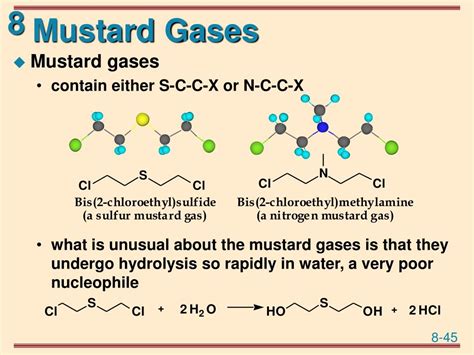
The chemical formula for mustard gas, also known as sulfur mustard, is C4H8Cl2S. This compound is a highly toxic and blistering agent that has been used as a chemical warfare agent in various conflicts. Mustard gas is classified as a vesicant, which means it causes severe burns and blisters on the skin and mucous membranes. The chemical structure of mustard gas consists of a sulfur atom bonded to two chlorine atoms and two ethyl groups, which are responsible for its toxic and corrosive properties.
Chemical Properties of Mustard Gas
Mustard gas is a colorless, odorless liquid at room temperature, but it can also exist as a vapor. It has a melting point of 14°C and a boiling point of 218°C. The compound is highly soluble in organic solvents, such as ethanol and chloroform, but it is relatively insoluble in water. Mustard gas is also highly reactive, and it can undergo various chemical reactions, including hydrolysis and oxidation, which can lead to the formation of toxic byproducts.
Synthesis of Mustard Gas
Mustard gas can be synthesized through the reaction of ethylene with sulfur dichloride, followed by the addition of chlorine gas. This process involves the formation of a intermediate compound, which is then converted to mustard gas through a series of chemical reactions. The synthesis of mustard gas is highly regulated, and it is only permitted for research and development purposes in certain countries.
| Chemical Property | Value |
|---|---|
| Molecular Formula | C4H8Cl2S |
| Molecular Weight | 159.08 g/mol |
| Boiling Point | 218°C |
| Melting Point | 14°C |
| Solubility in Water | 0.07 g/100 mL |
Key Points
- The chemical formula for mustard gas is C4H8Cl2S.
- Mustard gas is a highly toxic and blistering agent that has been used as a chemical warfare agent.
- The compound has a melting point of 14°C and a boiling point of 218°C.
- Mustard gas is highly soluble in organic solvents, but it is relatively insoluble in water.
- The synthesis of mustard gas is highly regulated, and it is only permitted for research and development purposes in certain countries.
Toxicity and Health Effects of Mustard Gas

Mustard gas is a highly toxic substance that can cause severe health effects, including burns, blisters, and respiratory problems. The compound can also cause long-term health effects, such as cancer and reproductive problems. The toxicity of mustard gas is due to its ability to alkylate DNA, which can lead to genetic mutations and cell death. The health effects of mustard gas can be treated with various medical interventions, including decontamination, wound care, and respiratory support.
Decontamination and Treatment of Mustard Gas Exposure
Decontamination is the first step in treating mustard gas exposure, and it involves the removal of the contaminated clothing and washing of the affected area with soap and water. Medical treatment for mustard gas exposure may include the administration of antidotes, such as dimercaprol, and the use of supportive care, such as wound care and respiratory support. The treatment of mustard gas exposure requires a multidisciplinary approach, and it involves the coordination of medical, nursing, and laboratory personnel.
What is the chemical formula for mustard gas?
+The chemical formula for mustard gas is C4H8Cl2S.
What are the health effects of mustard gas exposure?
+Mustard gas exposure can cause severe health effects, including burns, blisters, and respiratory problems. The compound can also cause long-term health effects, such as cancer and reproductive problems.
How is mustard gas synthesized?
+Mustard gas can be synthesized through the reaction of ethylene with sulfur dichloride, followed by the addition of chlorine gas.
Meta description: Learn about the chemical formula, properties, and toxicity of mustard gas, a highly toxic and blistering agent used as a chemical warfare agent.