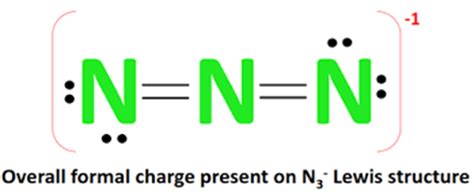The N3 Lewis structure, also known as the azide ion, is a fundamental concept in chemistry that represents the arrangement of electrons in a molecule. To understand the N3 Lewis structure, it's essential to have a basic knowledge of chemistry, particularly in the fields of molecular geometry and electron configuration. In this article, we will delve into the world of Lewis structures, exploring the N3 Lewis structure, its significance, and how to draw it.
Key Points
- The N3 Lewis structure consists of three nitrogen atoms bonded together, with a central nitrogen atom double-bonded to one of the terminal nitrogen atoms and single-bonded to the other.
- The azide ion has a total of 16 valence electrons, which are distributed among the three nitrogen atoms to form a stable molecule.
- The N3 Lewis structure is essential in understanding the chemical properties and reactivity of the azide ion.
- Drawing the N3 Lewis structure requires a step-by-step approach, starting with the central nitrogen atom and working outwards to the terminal nitrogen atoms.
- Understanding the N3 Lewis structure is crucial in various fields, including organic chemistry, inorganic chemistry, and materials science.
Introduction to Lewis Structures
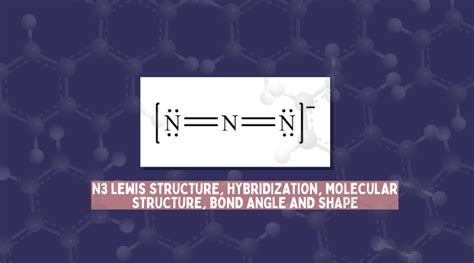
Lewis structures, also known as electron dot diagrams, are a graphical representation of the electron configuration of a molecule. They were first introduced by Gilbert N. Lewis in 1916 and have since become a fundamental tool in chemistry. Lewis structures help chemists visualize the arrangement of electrons in a molecule, which is essential in understanding the chemical properties and reactivity of the molecule.
What is the N3 Lewis Structure?
The N3 Lewis structure represents the azide ion, which consists of three nitrogen atoms bonded together. The central nitrogen atom is double-bonded to one of the terminal nitrogen atoms and single-bonded to the other. The azide ion has a total of 16 valence electrons, which are distributed among the three nitrogen atoms to form a stable molecule. The N3 Lewis structure is essential in understanding the chemical properties and reactivity of the azide ion.
| Atom | Valence Electrons |
|---|---|
| Nitrogen (central) | 5 |
| Nitrogen (terminal) | 5 |
| Nitrogen (terminal) | 5 |
| Total Valence Electrons | 16 |
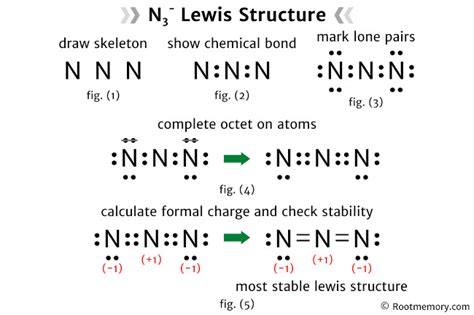
Drawing the N3 Lewis Structure
Drawing the N3 Lewis structure requires a step-by-step approach. The first step is to determine the central atom, which in this case is the nitrogen atom. The next step is to arrange the terminal nitrogen atoms around the central atom, making sure to satisfy the octet rule for each atom. The octet rule states that each atom should have eight electrons in its valence shell to be stable.
Once the terminal nitrogen atoms are in place, the next step is to distribute the valence electrons among the atoms. The central nitrogen atom should have a double bond with one of the terminal nitrogen atoms and a single bond with the other. The remaining electrons should be distributed as lone pairs on the terminal nitrogen atoms.
Importance of the N3 Lewis Structure
The N3 Lewis structure is essential in understanding the chemical properties and reactivity of the azide ion. The azide ion is a common ligand in coordination chemistry and is used in various applications, including the production of pharmaceuticals, agrochemicals, and materials. Understanding the N3 Lewis structure is crucial in designing and synthesizing new compounds with specific properties.
Applications of the N3 Lewis Structure
The N3 Lewis structure has various applications in chemistry and materials science. The azide ion is used as a ligand in coordination chemistry to form complexes with metal ions. These complexes have unique properties and are used in various applications, including catalysis, materials science, and pharmaceuticals.
The N3 Lewis structure is also used in organic chemistry to design and synthesize new compounds with specific properties. The azide ion is a common functional group in organic chemistry and is used in various reactions, including click chemistry and azide-alkyne cycloaddition.
What is the significance of the N3 Lewis structure?
+The N3 Lewis structure is significant because it helps us understand the chemical properties and reactivity of the azide ion. The azide ion is a common ligand in coordination chemistry and is used in various applications, including the production of pharmaceuticals, agrochemicals, and materials.
How is the N3 Lewis structure drawn?
+The N3 Lewis structure is drawn by determining the central atom, arranging the terminal nitrogen atoms around the central atom, and distributing the valence electrons among the atoms. The central nitrogen atom should have a double bond with one of the terminal nitrogen atoms and a single bond with the other.
What are the applications of the N3 Lewis structure?
+The N3 Lewis structure has various applications in chemistry and materials science. The azide ion is used as a ligand in coordination chemistry to form complexes with metal ions. These complexes have unique properties and are used in various applications, including catalysis, materials science, and pharmaceuticals.
In conclusion, the N3 Lewis structure is a fundamental concept in chemistry that represents the arrangement of electrons in the azide ion. Understanding the N3 Lewis structure is essential in understanding the chemical properties and reactivity of the azide ion, which has various applications in chemistry and materials science. By following the step-by-step approach to drawing the N3 Lewis structure, chemists can visualize the arrangement of electrons in the molecule and design new compounds with specific properties.