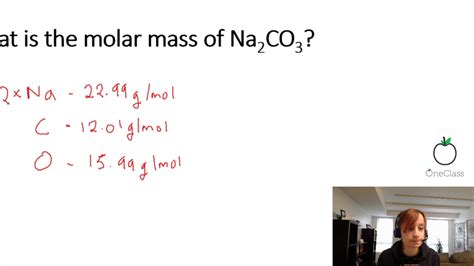Sodium carbonate, commonly known as washing soda or soda ash, is a chemical compound with the formula Na2CO3. Its molar mass is a fundamental physical property that is crucial in various chemical calculations, including stoichiometry and the preparation of solutions. The molar mass of Na2CO3 can be calculated by summing the atomic masses of its constituent elements: sodium (Na), carbon (C), and oxygen (O). Here, we'll delve into the calculation of the molar mass of Na2CO3 and explore its significance in chemical applications.
Key Points
- The molar mass of Na2CO3 is calculated by adding the atomic masses of two sodium atoms, one carbon atom, and three oxygen atoms.
- The atomic masses used for the calculation are approximately: Na = 22.99 g/mol, C = 12.01 g/mol, and O = 16.00 g/mol.
- The molar mass of Na2CO3 is essential in determining the amount of substance in chemical reactions and preparations.
- Understanding the molar mass of compounds like Na2CO3 is vital for accurate calculations in chemistry, ensuring the correct proportions of reactants are used.
- The application of molar mass in chemistry extends to fields like analytical chemistry, where it's used in titration calculations and the preparation of standard solutions.
Calculating the Molar Mass of Na2CO3

To calculate the molar mass of Na2CO3, we follow a straightforward process. First, we identify the atomic masses of sodium (Na), carbon ©, and oxygen (O) from the periodic table. The approximate atomic masses are: Na = 22.99 g/mol, C = 12.01 g/mol, and O = 16.00 g/mol. The formula Na2CO3 indicates there are two sodium atoms, one carbon atom, and three oxygen atoms in each molecule of sodium carbonate.
Step-by-Step Calculation
The calculation involves multiplying the atomic mass of each element by the number of atoms of that element in the compound and then summing these values. For Na2CO3, the calculation is as follows:
2 * (atomic mass of Na) + (atomic mass of C) + 3 * (atomic mass of O)
Substituting the atomic masses:
2 * 22.99 g/mol + 12.01 g/mol + 3 * 16.00 g/mol
This calculation yields:
2 * 22.99 g/mol = 45.98 g/mol
Adding 12.01 g/mol for carbon gives: 45.98 g/mol + 12.01 g/mol = 57.99 g/mol
Finally, adding 3 * 16.00 g/mol for oxygen: 57.99 g/mol + 48.00 g/mol = 105.99 g/mol
Thus, the molar mass of Na2CO3 is approximately 105.99 g/mol.
| Element | Atomic Mass (g/mol) | Number of Atoms | Total Mass Contribution |
|---|---|---|---|
| Sodium (Na) | 22.99 | 2 | 45.98 |
| Carbon (C) | 12.01 | 1 | 12.01 |
| Oxygen (O) | 16.00 | 3 | 48.00 |
| Total | 105.99 |
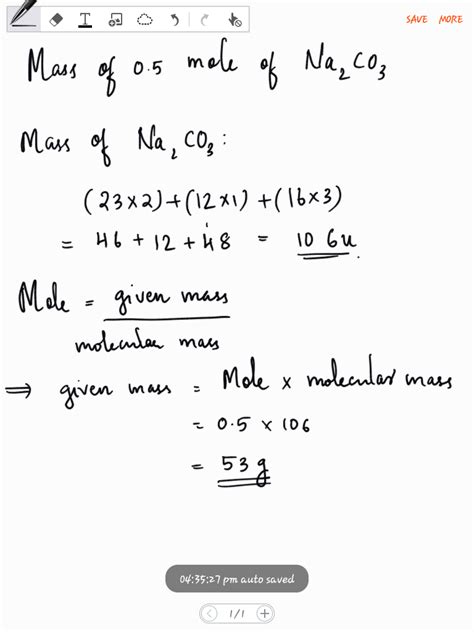
Significance of Molar Mass in Chemical Applications
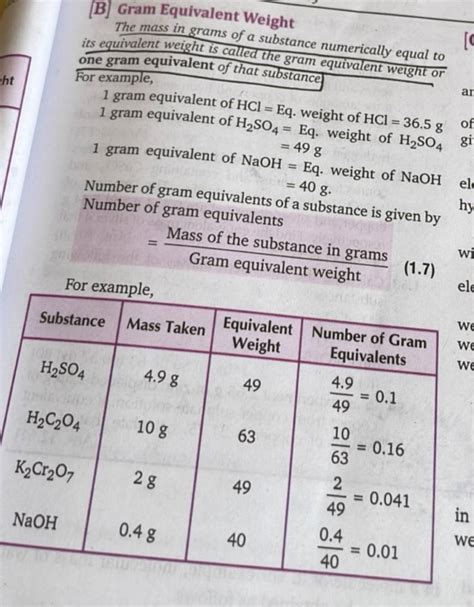
The molar mass of Na2CO3, like that of any other compound, is crucial for various chemical applications. In stoichiometry, the molar mass is used to relate the amounts of substances involved in chemical reactions. For instance, if a reaction requires a specific mole ratio of Na2CO3 to another reactant, knowing the molar mass of Na2CO3 allows chemists to calculate the exact mass of Na2CO3 needed to react with a given mass of the other reactant.
Applications in Solution Preparation
In the preparation of solutions, the molar mass of Na2CO3 is used to calculate the mass of the compound needed to achieve a desired molarity. Molarity is defined as the number of moles of solute per liter of solution. By knowing the molar mass of Na2CO3, one can calculate how much of the compound to dissolve in a given volume of solvent to achieve a specific molarity.
The applications of molar mass extend beyond the laboratory to industrial processes and environmental science. Understanding the molar masses of compounds like Na2CO3 is essential for managing chemical reactions on a large scale, ensuring safety, efficiency, and compliance with environmental regulations.
What is the primary use of molar mass in chemistry?
+The primary use of molar mass in chemistry is to calculate the number of moles of a substance, which is critical for understanding reaction stoichiometry and preparing solutions with accurate concentrations.
How is the molar mass of Na2CO3 calculated?
+The molar mass of Na2CO3 is calculated by summing the atomic masses of its constituent elements: two sodium atoms, one carbon atom, and three oxygen atoms.
What is the significance of knowing the molar mass of compounds like Na2CO3 in industrial processes?
+Knowing the molar mass of compounds like Na2CO3 is significant in industrial processes as it allows for the precise calculation of reactant amounts, ensuring efficiency, safety, and compliance with environmental regulations.
In conclusion, the molar mass of Na2CO3 is a fundamental property that underpins various chemical calculations and applications. Its accurate determination and application are crucial for stoichiometric calculations, solution preparation, and industrial processes. The significance of molar mass extends beyond the realm of chemistry, impacting fields such as environmental science and engineering. As such, understanding and accurately calculating the molar mass of compounds like Na2CO3 is essential for advancing chemical knowledge and applying it effectively in real-world scenarios.