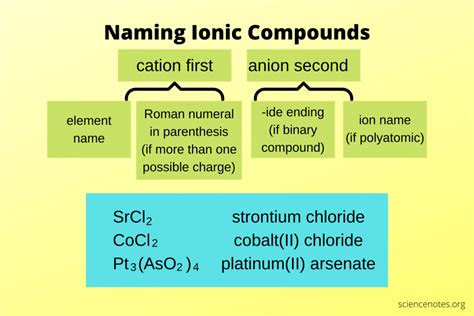
Naming ionic compounds is a fundamental concept in chemistry, and it can be made easy by following a few simple rules. Ionic compounds are formed when a metal atom loses one or more electrons to form a positively charged ion, known as a cation, and a non-metal atom gains one or more electrons to form a negatively charged ion, known as an anion. The combination of these ions results in the formation of an ionic compound. In this article, we will explore the rules for naming ionic compounds and provide examples to illustrate the process.
Key Points
- Ionic compounds are formed by the combination of cations and anions.
- The name of an ionic compound consists of the name of the cation followed by the name of the anion.
- Cations are named based on the name of the metal, while anions are named based on the name of the non-metal with a suffix of -ide.
- Subscripts are used to indicate the ratio of ions in the compound.
- Transition metals can have multiple charges, and their names must be modified to indicate the charge.
Naming Cations

Cations are named based on the name of the metal. For example, the cation formed from sodium is called sodium, and the cation formed from calcium is called calcium. Transition metals, however, can have multiple charges, and their names must be modified to indicate the charge. For example, the cation formed from iron can have a charge of +2 or +3, and its name is modified to iron(II) or iron(III), respectively.
Naming Anions
Anions are named based on the name of the non-metal with a suffix of -ide. For example, the anion formed from oxygen is called oxide, and the anion formed from chlorine is called chloride. If the non-metal has multiple possible charges, the name of the anion must be modified to indicate the charge. For example, the anion formed from sulfur can have a charge of -2 or -1, and its name is modified to sulfide or sulfite, respectively.
| Cation | Anion | Compound Name |
|---|---|---|
| Sodium (Na+) | Oxide (O2-) | Sodium oxide |
| Calcium (Ca2+) | Chloride (Cl-) | Calcium chloride |
| Iron(III) (Fe3+) | Sulfide (S2-) | Iron(III) sulfide |

Naming Ionic Compounds with Polyatomic Ions
Polyatomic ions are ions that consist of multiple atoms. They are named based on the name of the central atom with a prefix indicating the number of oxygen atoms. For example, the polyatomic ion NO3- is called nitrate, and the polyatomic ion SO42- is called sulfate. When naming ionic compounds that contain polyatomic ions, the name of the cation is written first, followed by the name of the polyatomic ion.
Examples of Ionic Compounds with Polyatomic Ions
Here are some examples of ionic compounds that contain polyatomic ions:
- Sodium nitrate (NaNO3)
- Calcium sulfate (CaSO4)
- Iron(III) phosphate (FePO4)
In each of these examples, the name of the cation is written first, followed by the name of the polyatomic ion. The subscripts are used to indicate the ratio of ions in the compound, but they are not included in the name of the compound.
What is the difference between a cation and an anion?
+A cation is a positively charged ion, while an anion is a negatively charged ion. Cations are formed when a metal atom loses one or more electrons, while anions are formed when a non-metal atom gains one or more electrons.
How do you name an ionic compound that contains a polyatomic ion?
+When naming an ionic compound that contains a polyatomic ion, the name of the cation is written first, followed by the name of the polyatomic ion. The subscripts are used to indicate the ratio of ions in the compound, but they are not included in the name of the compound.
What is the purpose of using subscripts in the formula of an ionic compound?
+Subscripts are used to indicate the ratio of ions in the compound. They are essential in writing the correct formula for an ionic compound.
In conclusion, naming ionic compounds can be made easy by following a few simple rules. The name of the cation is always written first, followed by the name of the anion. Polyatomic ions are named based on the name of the central atom with a prefix indicating the number of oxygen atoms. Subscripts are used to indicate the ratio of ions in the compound, but they are not included in the name of the compound. By mastering these rules, you can easily name ionic compounds and improve your understanding of chemistry.
Meta Description: Learn the rules for naming ionic compounds and how to apply them to different types of compounds. Discover how to name cations, anions, and polyatomic ions, and understand the importance of subscripts in the formula of an ionic compound.