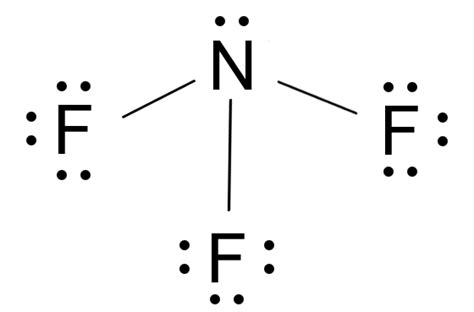
The chemical compound with the formula NF3 is known as nitrogen trifluoride. It is a colorless, toxic, and corrosive gas with a characteristic moldy odor. NF3 is used in various industrial applications, including as an etchant in the production of microelectronic devices, such as semiconductors and flat panel displays. Additionally, it serves as a cleaning agent for chemical vapor deposition (CVD) reactors and as a fluorinating agent in various chemical reactions.
Nitrogen Trifluoride Properties and Applications
Nitrogen trifluoride (NF3) exhibits several distinct physical and chemical properties that make it useful for specific applications. It has a molecular weight of 71.0019 g/mol, a boiling point of -129°C, and a melting point of -206.8°C. NF3 is highly reactive due to the strong electronegativity of fluorine, which creates a significant partial positive charge on the nitrogen atom. This reactivity is exploited in plasma etching processes for semiconductor manufacturing, where NF3 is used to remove silicon dioxide and silicon nitride layers.
Chemical Synthesis and Handling
The synthesis of NF3 typically involves the reaction of ammonia (NH3) with fluorine (F2) in the presence of a catalyst. However, this method poses significant safety risks due to the highly reactive nature of fluorine. An alternative, safer route involves the electrolysis of ammonium fluoride (NH4F) in anhydrous hydrogen fluoride (HF). NF3 is highly toxic and requires careful handling, including the use of personal protective equipment and specialized storage containers to prevent exposure and environmental release.
| Physical Property | Value |
|---|---|
| Molecular Weight | 71.0019 g/mol |
| Boiling Point | -129°C |
| Melting Point | -206.8°C |
Key Points
- Nitrogen trifluoride (NF3) is a toxic and corrosive gas used in various industrial applications, including semiconductor manufacturing and chemical vapor deposition processes.
- Its high reactivity is due to the electronegativity of fluorine, creating a partial positive charge on the nitrogen atom.
- Synthesis methods include the reaction of ammonia with fluorine and the electrolysis of ammonium fluoride in anhydrous hydrogen fluoride.
- Handling NF3 requires strict safety measures due to its toxicity and reactivity.
- Applications in microelectronic device production highlight its importance in modern technology.
Environmental and Safety Considerations
Beyond its industrial applications, NF3 has significant environmental and safety implications. It is recognized as a potent greenhouse gas, with a global warming potential (GWP) approximately 17,200 times that of carbon dioxide (CO2) over a 100-year time frame. This has led to increased scrutiny and regulatory efforts aimed at reducing emissions and promoting the development of more environmentally friendly alternatives. Additionally, the toxic nature of NF3 necessitates rigorous safety protocols to prevent occupational exposure and environmental contamination.
Regulatory Frameworks and Future Directions
Regulatory bodies, such as the United States Environmental Protection Agency (EPA) and the European Union’s climate and energy policy frameworks, have begun to address the environmental impact of NF3 through emission reduction targets and the promotion of sustainable technologies. As the electronics and semiconductor industries continue to evolve, there is a growing need for innovative, less harmful alternatives to NF3. Research into new etching technologies and fluorine-free processes is underway, driven by both environmental concerns and the quest for more efficient manufacturing processes.
What are the primary industrial applications of NF3?
+NF3 is primarily used in the production of microelectronic devices, such as semiconductors and flat panel displays, as an etchant and in cleaning chemical vapor deposition reactors.
Why is NF3 considered a potent greenhouse gas?
+NF3 has a global warming potential approximately 17,200 times that of CO2 over a 100-year time frame due to its long atmospheric lifetime and high infrared absorption capacity.
What safety measures are necessary when handling NF3?
+Handling NF3 requires the use of personal protective equipment, including gloves, safety glasses, and a face mask, as well as working in a well-ventilated area or fume hood to prevent exposure.
In conclusion, nitrogen trifluoride (NF3) is a complex and multifaceted compound with significant industrial applications and environmental implications. Its unique properties make it an essential component in the manufacture of microelectronic devices, but its toxic and greenhouse gas characteristics necessitate careful handling and stringent regulatory oversight. As technology continues to evolve, the development of safer, more sustainable alternatives to NF3 will be crucial for mitigating its environmental impact while advancing industrial capabilities.