The NH3 Lewis dot structure, also known as the ammonia molecule, is a fundamental concept in chemistry that represents the arrangement of electrons in a molecule. To understand the NH3 Lewis dot structure, it's essential to have a basic knowledge of chemistry and the rules that govern the formation of molecules. In this article, we'll delve into the world of NH3, exploring its Lewis dot structure, bonding, and properties.
Introduction to NH3 and Lewis Dot Structures
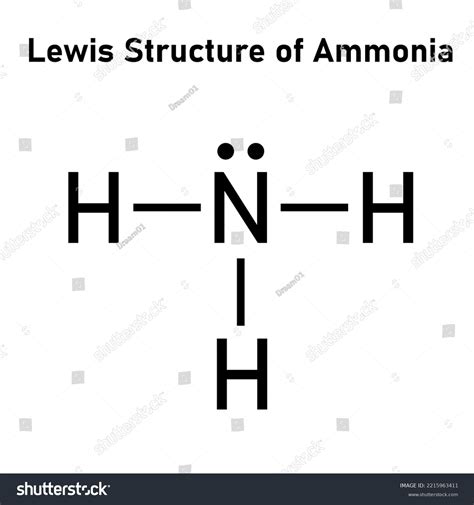
NH3, or ammonia, is a chemical compound composed of one nitrogen atom and three hydrogen atoms. The Lewis dot structure is a graphical representation of the molecule, showing the arrangement of electrons and bonds between atoms. The Lewis dot structure is a powerful tool for understanding the properties and behavior of molecules, and it’s widely used in chemistry and related fields. To create a Lewis dot structure, we need to follow a set of rules that ensure the molecule is stable and satisfies the octet rule, which states that each atom should have eight electrons in its outermost energy level.
Key Points
- The NH3 Lewis dot structure consists of one nitrogen atom and three hydrogen atoms.
- The nitrogen atom has five valence electrons, while each hydrogen atom has one valence electron.
- The molecule has a trigonal pyramidal shape, with the nitrogen atom at the center and the hydrogen atoms at the corners.
- The NH3 molecule has a polar bond, with the nitrogen atom having a slightly negative charge and the hydrogen atoms having a slightly positive charge.
- The Lewis dot structure of NH3 is essential for understanding its properties and behavior.
Drawing the NH3 Lewis Dot Structure
To draw the NH3 Lewis dot structure, we start by placing the nitrogen atom at the center and the three hydrogen atoms around it. The nitrogen atom has five valence electrons, which are represented by dots around the atom. Each hydrogen atom has one valence electron, which is also represented by a dot. We then draw single bonds between the nitrogen atom and each of the hydrogen atoms, using a line to represent the shared pair of electrons. The resulting structure shows the nitrogen atom with three single bonds and one lone pair of electrons, which is the characteristic feature of the NH3 molecule.
| Atom | Valence Electrons | Bonds |
|---|---|---|
| Nitrogen | 5 | 3 single bonds, 1 lone pair |
| Hydrogen | 1 | 1 single bond |

Properties and Behavior of NH3
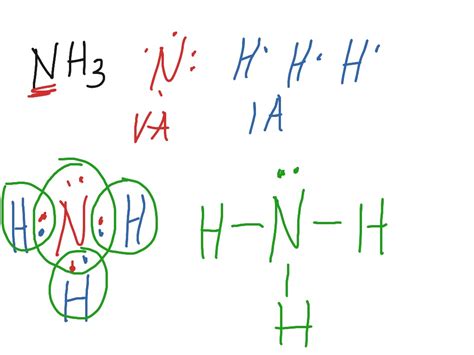
The NH3 molecule has several interesting properties and behaviors that are influenced by its Lewis dot structure. One of the most notable properties is its polarity, which arises from the difference in electronegativity between the nitrogen and hydrogen atoms. The nitrogen atom has a slightly negative charge, while the hydrogen atoms have a slightly positive charge, resulting in a polar bond. This polarity makes NH3 a polar molecule, which is essential for its solubility in water and its ability to form hydrogen bonds.
Bonding and Hybridization
The bonding in NH3 involves the overlap of atomic orbitals between the nitrogen and hydrogen atoms. The nitrogen atom has a trigonal pyramidal shape, with the three hydrogen atoms bonded to it through single bonds. The resulting molecule has a bent or V-shape, with the nitrogen atom at the center and the hydrogen atoms at the corners. The hybridization of the nitrogen atom is sp3, which means that it has four equivalent hybrid orbitals that are directed towards the corners of a tetrahedron. The hybridization of the hydrogen atoms is sp3, which means that they have one hybrid orbital that is directed towards the nitrogen atom.
What is the shape of the NH3 molecule?
+The NH3 molecule has a trigonal pyramidal shape, with the nitrogen atom at the center and the hydrogen atoms at the corners.
What is the polarity of the NH3 molecule?
+The NH3 molecule is polar, with the nitrogen atom having a slightly negative charge and the hydrogen atoms having a slightly positive charge.
What is the hybridization of the nitrogen atom in NH3?
+The hybridization of the nitrogen atom in NH3 is sp3, which means that it has four equivalent hybrid orbitals that are directed towards the corners of a tetrahedron.
In conclusion, the NH3 Lewis dot structure is a fundamental concept in chemistry that represents the arrangement of electrons in the ammonia molecule. Understanding the properties and behavior of NH3 is crucial for many applications, including the production of fertilizers, pharmaceuticals, and cleaning products. By analyzing the Lewis dot structure, bonding, and hybridization of NH3, we can gain a deeper understanding of its properties and behavior, and appreciate the importance of this molecule in our daily lives.


