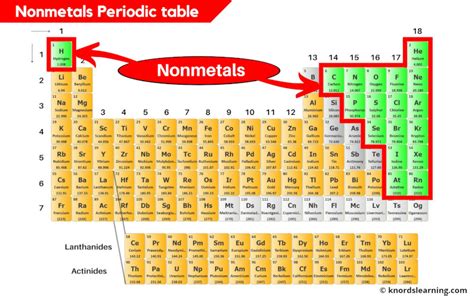
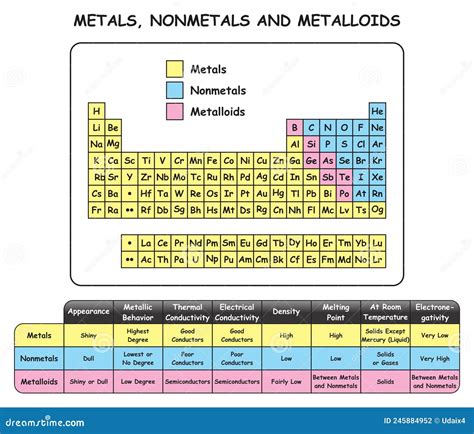
The periodic table, a fundamental tool in chemistry, is a tabular display of the known chemical elements, organized by their atomic number (number of protons in the nucleus), electron configuration, and recurring chemical properties. The elements are listed in order of increasing atomic number and are grouped into rows called periods and columns called groups or families. Among these elements, nonmetals occupy a significant portion, primarily located in the upper right corner of the periodic table. Nonmetals are a class of elements that, in contrast to metals, do not exhibit the characteristic properties of metals, such as luster, malleability, and the ability to conduct electricity and heat. Instead, nonmetals are typically dull, brittle, and poor conductors of electricity and heat.
Characteristics and Properties of Nonmetals
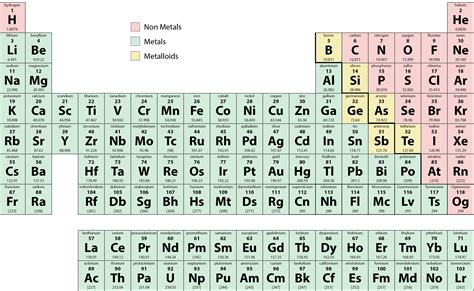
Nonmetals display a wide range of properties, but they generally share certain characteristics. They are often gases at room temperature, such as oxygen (O2), nitrogen (N2), and the noble gases (e.g., helium, neon, argon). Some nonmetals, like carbon (in the form of diamond or graphite) and sulfur, are solids. Bromine is the only nonmetal that is a liquid at room temperature. Nonmetals tend to gain electrons to form anions, which have a negative charge, when they react with other elements. This is in contrast to metals, which tend to lose electrons to form cations with a positive charge. The nonmetal elements also exhibit a wide range of electronegativities, a measure of an atom’s ability to attract electrons in a covalent bond. The electronegativity of nonmetals is generally higher than that of metals, reflecting their tendency to attract electrons.
Classification and Types of Nonmetals
Nonmetals can be broadly classified based on their position in the periodic table and their chemical properties. The most common nonmetals include hydrogen (H), carbon ©, nitrogen (N), oxygen (O), fluorine (F), neon (Ne), phosphorus (P), sulfur (S), chlorine (Cl), argon (Ar), selenium (Se), bromine (Br), krypton (Kr), iodine (I), xenon (Xe), radon (Rn), and the noble gases. Hydrogen, although placed with the metals in the periodic table, behaves as a nonmetal under many circumstances. The noble gases are a special group of nonmetals that are chemically inert under normal conditions due to their full outer energy level, which makes them stable and unreactive.
Key Points
- The nonmetals are located primarily in the upper right corner of the periodic table.
- Nonmetals tend to gain electrons to form negatively charged ions (anions) when they react.
- They exhibit a wide range of properties, including being gases, solids, or, in one case, a liquid at room temperature.
- Nonmetals are generally dull, brittle, and poor conductors of electricity and heat.
- The electronegativity of nonmetals is higher than that of metals, indicating a greater tendency to attract electrons in covalent bonds.
Chemical Behavior of Nonmetals
Nonmetals exhibit a variety of chemical behaviors, largely due to their electron configuration. Many nonmetals form covalent compounds, where they share electrons with other atoms to form molecules. For example, oxygen and hydrogen form water (H2O), a compound essential for life, through covalent bonding. Nonmetals can also form ionic bonds with metals, where electrons are transferred from the metal to the nonmetal, resulting in the formation of ions with opposite charges that are attracted to each other. The reaction of nonmetals with other elements can produce acids, bases, and a wide array of compounds that are crucial in biological and industrial processes.
Biological and Industrial Importance of Nonmetals
The importance of nonmetals cannot be overstated, as they are fundamental components of life and critical for many industrial processes. Oxygen is essential for the process of cellular respiration in most living organisms, where it acts as the final electron acceptor, allowing cells to produce energy. Carbon, in its various forms (such as carbohydrates, fats, proteins, and DNA), is the basis of all life on Earth. Nitrogen is a key component of amino acids, which are the building blocks of proteins. Phosphorus is crucial for the formation of DNA, RNA, and the molecule ATP (adenosine triphosphate), which provides energy for cellular processes. In industry, nonmetals are used in the production of fertilizers (e.g., ammonia, nitric acid), plastics (derived from carbon and hydrogen), and electronics (where silicon, a metalloid, plays a crucial role but works in conjunction with nonmetals).
| Nonmetal Element | Atomic Number | Common Uses |
|---|---|---|
| Carbon (C) | 6 | Biological molecules, fossil fuels, diamonds, graphite |
| Nitrogen (N) | 7 | Fertilizers, lasers, lighting |
| Oxygen (O) | 8 | Respiration, steel production, water treatment |
| Fluorine (F) | 9 | Dental health (toothpaste), refrigerants, semiconductors |
| Neon (Ne) | 10 | Lighting (neon signs), lasers |

Conclusion and Future Perspectives
In conclusion, nonmetals are a diverse group of elements that play critical roles in the natural world and in human society. Their unique properties and chemical behaviors make them essential for life and for the production of a wide range of materials and technologies. As research continues to uncover new properties and applications of nonmetals, their importance is likely to grow. The study of nonmetals and their compounds will remain a vibrant area of scientific inquiry, with potential breakthroughs in fields such as energy storage, biotechnology, and advanced materials.
What distinguishes nonmetals from metals in the periodic table?
+Nonmetals are distinguished from metals by their lack of luster, brittleness, and poor conductivity of electricity and heat. They also tend to gain electrons to form anions, in contrast to metals, which lose electrons to form cations.
What are some common uses of nonmetals in industry and biology?
+Nonmetals have a wide range of applications. In industry, they are used in the production of fertilizers, plastics, and electronics. Biologically, they form the basis of life, with carbon being the fundamental element of biological molecules, oxygen essential for respiration, and nitrogen critical for amino acids and thus proteins.
How do the properties of nonmetals contribute to their role in environmental and energy issues?
+The properties of nonmetals, such as their ability to form covalent bonds and their high electronegativities, make them crucial in environmental processes (like the carbon cycle and oxygen production) and in the development of new energy technologies (such as solar cells and fuel cells), where nonmetals like silicon and carbon play significant roles.